

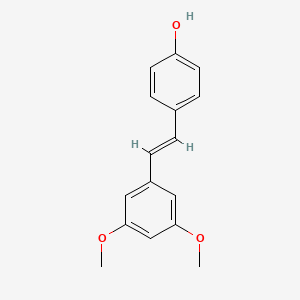

1. 3',5'-dimethoxy-4e-stilbenol
2. 3',5'-dimethoxy-4trans-stilbenol
3. 3',5'-dimethoxy-resveratrol
4. 3,5-dimethoxy-4'-hydroxy-trans-stilbene
5. 4-((e)-2-(3,5-dimethoxyphenyl)ethenyl)phenol
6. 4trans-(2-(3,5-dimethoxyphenyl)ethenyl)phenol
7. Phenol, 4-((1e)-2-(3,5-dimethoxyphenyl)ethenyl)-
8. Pterostilbene, (e)-
9. Trans-3,5-dimethoxy-4'-hydroxystilbene
10. Trans-pterostilbene
1. 537-42-8
2. Trans-pterostilbene
3. 4-(3,5-dimethoxystyryl)phenol
4. (e)-4-(3,5-dimethoxystyryl)phenol
5. 4-[(e)-2-(3,5-dimethoxyphenyl)ethenyl]phenol
6. 18259-15-9
7. 3',5'-dimethoxy-4-stilbenol
8. 4-[(e)-2-(3,5-dimethoxyphenyl)vinyl]phenol
9. Pterostilbene, (e)-
10. Pterostilben
11. 3,5-dimethoxy-4'-hydroxy-trans-stilbene
12. Trans-3,5-dimethoxy-4'-hydroxystilbene
13. Phenol, 4-[(1e)-2-(3,5-dimethoxyphenyl)ethenyl]-
14. Chembl83527
15. Chebi:8630
16. 4-((e)-2-(3,5-dimethoxyphenyl)ethenyl)phenol
17. 26r60s6a5i
18. 4-[(1e)-2-(3,5-dimethoxyphenyl)ethenyl]phenol
19. 3,5-dimethoxy-4'-hydroxystilbene
20. Phenol, 4-[2-(3,5-dimethoxyphenyl)ethenyl]-
21. Phenol, 4-((1e)-2-(3,5-dimethoxyphenyl)ethenyl)-
22. Mfcd00238710
23. Smr000440694
24. Pterostilbene, Pterocarpus Marsupium
25. 4-((1e)-2-(3,5-dimethoxyphenyl)ethenyl)phenol
26. Pteropure
27. Unii-26r60s6a5i
28. Tero-still-bean
29. 4'-hydroxy-3,5-dimethoxy-trans-stilbene
30. Cpd000440694
31. 4-stilbenol, 3',5'-dimethoxy-, (e)-
32. Phenol, 4-[(1z)-2-(3,5-dimethoxyphenyl)ethenyl]-
33. 3,5-di-o-methylresveratrol
34. Schembl20063
35. Mls000759434
36. Mls000863581
37. Mls001424135
38. Schembl563218
39. Pterostilbene [who-dd]
40. Gtpl2681
41. Megxp0_000345
42. Dtxsid9041106
43. Acon1_000305
44. 4'-hydroxy-3,5-dimethoxystilbene
45. Hms2051b10
46. Hms2270j16
47. Trans-1-(3,5-dimethoxyphenyl)-2-(4-hydroxyphenyl)ethylene
48. Zinc899213
49. Act03896
50. Albb-013778
51. Amy24840
52. Bcp22675
53. Hy-n0828
54. (e)-3',5'-dimethoxy-4-stilbenol
55. Bdbm50131688
56. Lmpk13090015
57. Nsc613735
58. S3937
59. 4''-hydroxy-3,5-dimethoxy Stilbene
60. Akos000277651
61. Akos025310510
62. Ac-5283
63. Ccg-101015
64. Cs-w008773
65. Eh-301 Component Pterostilbene
66. Nc00265
67. Nsc-613735
68. (e)-4'-hydroxy-3,5-dimethoxystilbene
69. Pterostilbene, >=97% (hplc), Solid
70. Ncgc00180691-01
71. As-13710
72. Ls-14484
73. 3,5-dimethoxy-4''-hydroxyl-trans-stilbene
74. Bb 0262300
75. N2126
76. P1924
77. 4-[2-(3,5-dimethoxy-phenyl)-vinyl]-phenol
78. Phenol,4-[2-(3,5-dmethoxyphenyl)ethenyl]-
79. (e)-1-hydroxy-4-(3,5-dimethoxy)styrylbenzene
80. E 4-[2-(3,5-dimethoxy-phenyl)-vinyl]-phenol
81. 259p159
82. 4-[(e)-2-(3,5-dimethoxy-phenyl)-vinyl]-phenol
83. A829757
84. J-523927
85. Phenol, 4-[(e)-2-(3,5-dimethoxyphenyl)ethenyl]-
86. Q2908011
87. W-203025
88. Phenol, 4-(2-(3,5-dimethoxyphenyl)ethenyl)-, (e)-
89. 4-[(e)-2-(3,5-dimethoxyphenyl)vinyl]phenol;pterostilbene
90. Ncgc00180691-03!4-[(e)-2-(3,5-dimethoxyphenyl)ethenyl]phenol
| Molecular Weight | 256.30 g/mol |
|---|---|
| Molecular Formula | C16H16O3 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 256.109944368 g/mol |
| Monoisotopic Mass | 256.109944368 g/mol |
| Topological Polar Surface Area | 38.7 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Pterostilbene has known human metabolites that include (2S,3S,4S,5R)-6-[4-[(E)-2-(3,5-dimethoxyphenyl)ethenyl]phenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560