

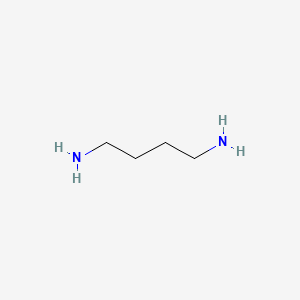

1. 1,4 Butanediamine
2. 1,4 Diaminobutane
3. 1,4-butanediamine
4. Putrescine
5. Tetramethylenediamine
1. Putrescine
2. 1,4-butanediamine
3. 110-60-1
4. Butane-1,4-diamine
5. Tetramethylenediamine
6. Butylenediamine
7. Putrescin
8. 1,4-butylenediamine
9. Tetramethyldiamine
10. 1,4-tetramethylenediamine
11. Putrescina
12. Putreszin
13. Tetramethylendiamin
14. Mfcd00008235
15. Nsc 60545
16. Brn 0605282
17. H2n(ch2)4nh2
18. Ai3-25444
19. 1,4-diamino-n-butane
20. Nsc-60545
21. Putrescine; Nsc 60545; Putramine
22. Chembl46257
23. V10tvz52e4
24. Chebi:17148
25. Put
26. Ccris 6751
27. Einecs 203-782-3
28. Unii-v10tvz52e4
29. Butylene Amine
30. Hsdb 7865
31. Tetramethylendiamine
32. 4-amino-butyl-amine
33. Tetramethylene Diamine
34. 1,4 Diamino Butane
35. 1,4-butane Diamine
36. 1,4-diamino Butane
37. Putrescine, Free Base
38. Spectrum_001646
39. 1i7c
40. 1i7m
41. Putrescine [mi]
42. Spectrum2_001935
43. Spectrum3_001198
44. Spectrum4_000237
45. Spectrum5_001005
46. Lopac-p-7505
47. Bmse000109
48. Bmse000814
49. Bmse000862
50. Ec 203-782-3
51. 1,4-diaminobutane, 99%
52. Butane,1,4-diamino
53. Lopac0_000972
54. Bspbio_002875
55. Kbiogr_000933
56. Kbioss_002126
57. Divk1c_000716
58. Spbio_001969
59. .alpha.,.omega.-butanediamine
60. 1,4-diaminobutane-[13c4]
61. Putrescine, Analytical Standard
62. Gtpl2388
63. Dtxsid4041107
64. Kbio1_000716
65. Kbio2_002126
66. Kbio2_004694
67. Kbio2_007262
68. Kbio3_002375
69. 1a99
70. Ninds_000716
71. Hy-n2407
72. Nsc60545
73. Zinc5828633
74. Bbl027703
75. Bdbm50009385
76. S5825
77. Stl372697
78. Akos000119071
79. Ccg-205052
80. Db01917
81. Sdccgmls-0066929.p001
82. Sdccgsbi-0050945.p003
83. Idi1_000716
84. Ncgc00015837-01
85. Ncgc00015837-02
86. Ncgc00015837-03
87. Ncgc00015837-07
88. Ncgc00162302-01
89. Bp-21408
90. Nci60_004431
91. Cs-0022608
92. D0239
93. Ft-0606836
94. 1,4-diaminobutane, Purum, >=98.0% (gc)
95. C00134
96. C02896
97. F17678
98. P-7990
99. 1,4-diaminobutane, Puriss., >=99.0% (gc)
100. Q410190
101. F1791-1258
102. Z1245649995
103. A4738f6f-f1a1-4b30-bf38-866d5ac66c93
104. 58i
| Molecular Weight | 88.15 g/mol |
|---|---|
| Molecular Formula | C4H12N2 |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 88.100048391 g/mol |
| Monoisotopic Mass | 88.100048391 g/mol |
| Topological Polar Surface Area | 52 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 17.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
The urinary excretion of histamine, methylhistamine, putrescine, cadaverine, spermidine and spermine was examined before, during and after pregnancy in rats. During the last third of undisturbed pregnancy a distinct and steep rise occurred in the excretion of all amines studied except spermine. The peak values were found a few days before the birth of the young. In spermidine excretion a second peak was observed one or two days after delivery. Before and during the first 2 weeks of gestation on a molar basis putrescine excretion was the greatest one. During the last trimester histamine was excreted in the largest amount. Under the influence of the diamine oxidase inhibitor aminoguanidine the general pattern of excretion of diamines and polyamines in pregnant rats remained essentially unchanged but the total amount excreted increased. Most conspicuous was the great elevation of urinary contents of putrescine and cadaverine.
PMID:745086 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1281758 Andersson AC et al; J Physiol 285: 311-24 (1978)
Putrescine is synthesized biologically via two different pathways, both starting from arginine. In one pathway, arginine is converted into agmatine, with a reaction catalyzed by the enzyme arginine decarboxylase (ADC); then agmatine is transformed into carbamilputrescine by agmatine imino hydroxylase (AIH). Finally, carbamilputrescine is converted into putrescine. In the second pathway, arginine is converted into ornithine and then ornithine is converted into putrescine by ornithine decarboxylase (ODC).
ChemieDe; Putresine. In Encyclopedia of Chemistry. Available from, as of November 19, 2010: https://www.chemie.de/lexikon/e/Putrescine/
Putrescine attacks s-adenosyl methionine and converts it to spermidine. Spermidine in turn ... /reacts with/ attacks another s-adenosyl methionine and converts it to spermine.
ChemieDe; Putresine. In Encyclopedia of Chemistry. Available from, as of November 19, 2010: https://www.chemie.de/lexikon/e/Putrescine/
Putrescine is synthesized in small quantities by healthy living cells by the action of ornithine decarboxylase. The polyamines, of which putrescine is one of the simplest, appear to be growth factors necessary for cell division.
ChemieDe; Putresine. In Encyclopedia of Chemistry. Available from, as of November 19, 2010: https://www.chemie.de/lexikon/e/Putrescine/
A novel bacterial putrescine utilization pathway was discovered. Seven genes, the functions of whose products were not known, are involved in this novel pathway. Five of them encode enzymes that catabolize putrescine; one encodes a putrescine importer, and the other encodes a transcriptional regulator. This novel pathway involves six sequential steps as follows: 1) import of putrescine; 2) ATP-dependent gamma-glutamylation of putrescine; 3) oxidization of gamma-glutamylputrescine; 4) dehydrogenation of gamma-glutamyl-gamma-aminobutyraldehyde; 5) hydrolysis of the gamma-glutamyl linkage of gamma-glutamyl-gamma-aminobutyrate; and 6) transamination of gamma-aminobutyrate to form the final product of this pathway, succinate semialdehyde, which is the precursor of succinate.
PMID:15590624 Kurihara S et al; J Biol Chem 280 (6): 4602-8 (2005)