
1. Tisamid
1. Pyrazinecarboxamide
2. 98-96-4
3. Pyrazine-2-carboxamide
4. Zinamide
5. Pyrazinoic Acid Amide
6. 2-pyrazinecarboxamide
7. Aldinamide
8. Pirazinamid
9. Aldinamid
10. Tebrazid
11. Unipyranamide
12. Farmizina
13. Pirazimida
14. Eprazin
15. Novamid
16. Pyrafat
17. Pyrazine Carboxylamide
18. Pyrazineamide
19. Isopas
20. 2-carbamylpyrazine
21. Pyrazinecarboxylic Acid Amide
22. Pyrazinamidum
23. Pyrazide
24. Rozide
25. Pirazinamide
26. Pyrazine Carboxamide
27. D-50
28. Pyramizade
29. Mk 56
30. Pza
31. Nci-c01785
32. Mfcd00006132
33. Nsc 14911
34. .alpha.-pyrazinamide
35. T 165
36. Pirazinamida
37. Nsc-14911
38. Tisamid
39. 2kni5n06ti
40. Mls000069730
41. Pezetamid
42. Piraldina
43. Chebi:45285
44. Nsc14911
45. Pyrazinamide (pyrazinoic Acid Amide)
46. Ncgc00015833-09
47. Pyrazinamdie
48. Smr000036662
49. Pirazinamide [dcit]
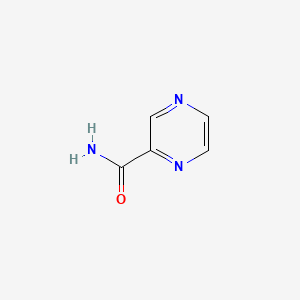
50. C5h5n3o
51. Dsstox_cid_1215
52. Dsstox_rid_76014
53. Dsstox_gsid_21215
54. Pyrazinamidum [inn-latin]
55. Pirazinamida [inn-spanish]
56. Rifafour
57. D-50 (van)
58. Drg 0124
59. Cas-98-96-4
60. Ccris 545
61. Pyrazinamide (tn)
62. Rifafour E-200
63. Hsdb 3576
64. Sr-01000076077
65. Einecs 202-717-6
66. Unii-2kni5n06ti
67. Brn 0112306
68. Pyrazinamida
69. Pyrizinamide
70. Pyrazine Amide
71. Azt + Pyrazinamide Combination
72. Pyrazine-2-carboxylic Acid Amide
73. Pyrazinamide,(s)
74. Pyrazinamide [usp:inn:ban:jan]
75. Prestwick_811
76. Pyranzinoic Acid Amide
77. 2-pyrazine Carboxamide
78. Spectrum_000902
79. Opera_id_735
80. Prestwick0_000514
81. Prestwick1_000514
82. Prestwick2_000514
83. Prestwick3_000514
84. Spectrum2_001305
85. Spectrum3_001046
86. Spectrum4_001186
87. Spectrum5_001026
88. Lopac-p-7136
89. Pyrazinamide [mi]
90. Pyrazinamide [inn]
91. Pyrazinamide [jan]
92. Chembl614
93. P 7136
94. Pyrazinamide [hsdb]
95. Wln: T6n Dnj Bvz
96. Pyrazinamide [vandf]
97. Pyrazine-2-carboximidic Acid
98. Lopac0_001011
99. Schembl24102
100. Bspbio_000467
101. Bspbio_002572
102. Kbiogr_001851
103. Kbioss_001382
104. Pyrazinamide [mart.]
105. 5-25-04-00178 (beilstein Handbook Reference)
106. Mls002222347
107. Bidd:gt0228
108. Divk1c_000241
109. Pyrazinamide [usp-rs]
110. Pyrazinamide [who-dd]
111. Pyrazinamide [who-ip]
112. Spectrum1500518
113. Spbio_001369
114. Spbio_002388
115. Bpbio1_000515
116. Gtpl7287
117. Zinc2005
118. Dtxsid9021215
119. Hms500m03
120. Kbio1_000241
121. Kbio2_001382
122. Kbio2_003950
123. Kbio2_006518
124. Kbio3_001792
125. Pyrazinamide (jp17/usp/inn)
126. Ninds_000241
127. Bdbm228814
128. Hms1569h09
129. Hms1920n08
130. Hms2092e09
131. Hms2096h09
132. Hms2235g17
133. Hms3259o04
134. Hms3263k03
135. Hms3371g09
136. Hms3655a10
137. Hms3713h09
138. Kuc109577n
139. Pharmakon1600-01500518
140. Pyrazinamide [orange Book]
141. Pyrazinamide [ep Monograph]
142. Pyrazinamide [usp Impurity]
143. Act01761
144. Amy14180
145. Bcp30257
146. Hy-b0271
147. Ksc-27-052e
148. Pyrazine-[d3]-carboxamide-[15n]
149. Pyrazinamide [usp Monograph]
150. Tox21_110237
151. Tox21_202059
152. Tox21_302771
153. Tox21_501011
154. Ccg-39243
155. Nsc757304
156. Pyrazinamidum [who-ip Latin]
157. Rifater Component Pyrazinamide
158. S1762
159. Stk801661
160. Akos000120280
161. Tox21_110237_1
162. Db00339
163. Lp01011
164. Nc00534
165. Nsc-757304
166. Pyrazinecarboxamide, Analytical Standard
167. Sdccgsbi-0050984.p005
168. Idi1_000241
169. Pyrazinamide Component Of Rifater
170. Ncgc00015833-01
171. Ncgc00015833-02
172. Ncgc00015833-03
173. Ncgc00015833-04
174. Ncgc00015833-05
175. Ncgc00015833-06
176. Ncgc00015833-07
177. Ncgc00015833-08
178. Ncgc00015833-10
179. Ncgc00015833-11
180. Ncgc00015833-12
181. Ncgc00015833-15
182. Ncgc00015833-16
183. Ncgc00015833-25
184. Ncgc00090695-01
185. Ncgc00090695-03
186. Ncgc00090695-04
187. Ncgc00090695-05
188. Ncgc00090695-06
189. Ncgc00090695-07
190. Ncgc00256336-01
191. Ncgc00259608-01
192. Ncgc00261696-01
193. Pyrazinoic Acid Amide; Pyrazinamide; Pza
194. Cas- 98-96-4
195. Sy013550
196. Ts-01626
197. Sbi-0050984.p004
198. Db-002866
199. Ab00052083
200. B2122
201. Bb 0253141
202. Eu-0101011
203. Ft-0659757
204. P0633
205. Sw196945-3
206. C01956
207. D00144
208. D70481
209. 1,2-dihydro-1,2,4-triazol-3-one;pyrazinamide
210. Ab00052083-16
211. Ab00052083_17
212. Ab00052083_18
213. A845937
214. Ac-907/25014068
215. Q417571
216. Sr-01000076077-1
217. Sr-01000076077-4
218. Sr-01000076077-6
219. W-100059
220. Z33546644
221. Pyrazinamide, British Pharmacopoeia (bp) Reference Standard
222. Pyrazinamide, European Pharmacopoeia (ep) Reference Standard
223. 2-carbamylpyrazine ;aldinamid ;aldinamide; Pyrazinoic Acid Amide
224. Pyrazinamide, United States Pharmacopeia (usp) Reference Standard
225. Pyrazinamide, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 123.11 g/mol |
|---|---|
| Molecular Formula | C5H5N3O |
| XLogP3 | -0.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 123.043261792 g/mol |
| Monoisotopic Mass | 123.043261792 g/mol |
| Topological Polar Surface Area | 68.9 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 115 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Pyrazinamide |
| PubMed Health | Pyrazinamide (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | Pyrazinamide, the pyrazine analogue of nicotinamide, is an antituberculous agent. It is a white crystalline powder, stable at room temperature, and sparingly soluble in water. Pyrazinamide has the following structural formula:C5H5N3O M.W.123.11Each P... |
| Active Ingredient | Pyrazinamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Dava Pharms; Mikart |
| 2 of 2 | |
|---|---|
| Drug Name | Pyrazinamide |
| PubMed Health | Pyrazinamide (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | Pyrazinamide, the pyrazine analogue of nicotinamide, is an antituberculous agent. It is a white crystalline powder, stable at room temperature, and sparingly soluble in water. Pyrazinamide has the following structural formula:C5H5N3O M.W.123.11Each P... |
| Active Ingredient | Pyrazinamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Dava Pharms; Mikart |
Antitubercular Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Pyrazinamide is indicated in combination with other antimycobacterial drugs, in the treatment of tuberculosis. Pyrazinamide is effective only against mycobacteria. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2521
Rifampin, isoniazid, and pyrazinamide combination is indicated in the initial phase of the short-course treatment of all forms of tuberculosis. During this phase, which should last 2 months, rifampin, isoniazid, and pyrazinamide combination should be administered on a daily, continuous basis. Additional medications are indicated if multidrug-resistant tuberculosis is suspected. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2607
Pyrazinamide has become an important component of short-term (6 month) multiple-drug therapy of tuberculosis.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1281
Patients hypersensitive to ethionamide, isoniazid, niacin (nicotinic acid), or other chemically related medications may be hypersensitive to this medication also.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2521
Pyrazinamide should be used only when close observation of the patient is possible. Serum AST (SGOT), ALT (SGPT), and uric acid concentrations should be determined prior to and every 2-4 weeks during pyrazinamide therapy. If signs of hepatic damage occur, pyrazinamide should be discontinued.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 504
Hepatotoxicity is the most commonly reported adverse effect, with elevated transaminase levels being the earliest indication of toxicity...Pyrazinamide decreases the tubular excretion of uric acid, which may induce an acute gouty arthritis. Other adverse reactions include nausea, vomiting, dysuria, malaise, fever and skin rashes.
Ford MD, Delaney KA, Ling LJ, Erickson T; Clinical Toxicology. W.B. Saunders Company., Philadelphia, PA. 2001, p. 443
The drug inhibits excretion of urate, resulting in hyperuricemia in nearly all patients; acute episodes of gout have occurred. Other untoward effects that have been observed with pyrazinamide are arthralgias, anorexia, nausea and vomiting, dysuria, malaise and fever.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1282
For more Drug Warnings (Complete) data for PYRAZINAMIDE (19 total), please visit the HSDB record page.
For the initial treatment of active tuberculosis in adults and children when combined with other antituberculous agents.
Pyrazinamide kills or stops the growth of certain bacteria that cause tuberculosis (TB). It is used with other drugs to treat tuberculosis. It is a highly specific agent and is active only against Mycobacterium tuberculosis. In vitro and in vivo, the drug is active only at a slightly acid pH. Pyrazinamie gets activated to Pyrazinoic acid in the bacilli where it interferes with fatty acid synthase FAS I. This interferes with the bacteriums ability to synthesize new fatty acids, required for growth and replication.
Antitubercular Agents
Drugs used in the treatment of tuberculosis. They are divided into two main classes: "first-line" agents, those with the greatest efficacy and acceptable degrees of toxicity used successfully in the great majority of cases; and "second-line" drugs used in drug-resistant cases or those in which some other patient-related condition has compromised the effectiveness of primary therapy. (See all compounds classified as Antitubercular Agents.)
J04AK01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J04 - Antimycobacterials
J04A - Drugs for treatment of tuberculosis
J04AK - Other drugs for treatment of tuberculosis
J04AK01 - Pyrazinamide
Absorption
Rapidly and well absorbed from the gastrointestinal tract.
Route of Elimination
Approximately 70% of an oral dose is excreted in the urine, mainly by glomerular filtration within 24 hours
Pyrazinamide is well absorbed from the gastrointestinal tract, and it is widely distributed throughout the body. The oral administration of 500 mg produces plasma concentrations of about 9-12 ug/ml at two hours and 7 ug/ml at 8 hours.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1281
Pyrazinamide is well absorbed from the GI tract. Following a single 500 mg oral dose in healthy adults, peak plasma concentrations of pyrazinamide ranging from 9-12 ug/ml are attained within 2 hours; plasma concentrations of the drug average 7 ug/ml at 8 hours and 2 ug/ml at 24 hours. Plasma concentrations following doses of 20-25 mg/kg reportedly range from 3-50 ug/ml. Plasma concentrations of pyrazinoic acid, the major active metabolite of pyrazinamide, generally are greater than those of the parent drug and peak within 4-8 hours after an oral dose of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 508
In a single-dose study in healthy fasting males, the extent of absorption (as measured by area under the plasma concentration-time curve) of isoniazid, rifampin, or pyrazinamide in dosages of 250, 6O0, or 1500 mg, respectively, was similar whether the drugs were administered individually as capsules (rifampin) and tablets (isoniazid and pyrazinamide) or as a fixed combination containing isoniazid 50 mg, rifampin 120 mg, and pyrazinamide 300 mg per tablet.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 508
Pyrazinamide is widely distributed into body tissues and fluids including the liver, lungs, and CSF. In a limited number of adults with tuberculous meningitis, mean serum and CSF concentrations of pyrazinamide 2 hours after an oral dose of approximately 41 mg/kg were 52 and 39 ug/ml, respectively. Within 5 hours after an oral dose, CSF concentrations of pyrazinamide are reported to be approximately equal to concurrent plasma concentrations of the drug. Plasma protein binding of pyrazinamide (determined by ultrafiltration) in a limited number of healthy men averaged approximately 17% at a pyrazinamide concentration of 20 ug/ml. It is not known if pyrazinamide crosses the placenta. It is not known if pyrazinamide is distributed into milk.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 508
For more Absorption, Distribution and Excretion (Complete) data for PYRAZINAMIDE (12 total), please visit the HSDB record page.
Hepatic.
Pyrazinamide is hydrolyzed to pyrazinoic acid and subsequently hydroxylated to 5-hydroxypyrazinoic acid, the major excretory product.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1281
The major metabolic pathway of pyrazinamide is conversion to pyrazinoic acid followed by subsequent conversion to hydroxypyrazinoic acid, a reaction catalyzed by xanthine oxidase.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 1645
Eight healthy volunteers were treated with a single dose of pyrazinamide 35 mg/kg. The aim of the study was to evaluate the pharmacokinetic profile of the product and of its metabolites. Urine and blood samples were collected till the 60th h. The kinetics of pyrazinamide could be characterized as follows: CPmax = 50.1 micrograms/ml, tmax less than 1 h, t1/2 alpha = 3.2 h, t1/2 beta = 23 h, U(0-60 h) = 1.6% of the dose administered. The kinetics of the main metabolite, the pyrazinoic acid, gave the following values: CPmax = 66.6 micrograms/ml, tmax = 4 h, t1/2 beta = 12.3 h, U(0-60 h) = 37.5%, of the administered dose.
Bareggi S et al; Arzeimittelforschung 37(7): p.849-854 (1987)
All available data support the idea that the PZA metabolite pyrazinoic acid (PA) is the active compound against M. tuberculosis. ... Caffeine, which is widely used as a drug and is a common constituent of most diets, shares with PZA the same metabolic enzyme, xanthine oxidase (XO).
Mehmedagic A et al; Biopharm Drug Dispos 23(5): p.191-195 (2002)
9-10 hours (normal conditions)
The plasma half-life is 9-10 hours in patients with normal renal function.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1281
The half-life of pyrazinamide is 23 hours. ... The elimination half-life is 10 to 16 hours.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 1645
The plasma half-life of pyrazinamide is 9-10 hours in patients with normal renal and hepatic function. The plasma half-life of the drug may be prolonged in patients with impaired renal or hepatic function.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 508
Pyrazinamide diffuses into active _M. tuberculosis_ that express pyrazinamidase enzyme that converts pyrazinamide to the active form pyrazinoic acid. Pyrazinoic acid can leak out under acidic conditions to be converted to the protonated conjugate acid, which is readily diffused back into the bacilli and accumulate intracellularly. The net effect is that more pyrazinoic acid accumulates inside the bacillus at acid pH than at neutral pH. Pyrazinoic acid was thought to inhibit the enzyme fatty acid synthase (FAS) I, which is required by the bacterium to synthesise fatty acids. However, this theory was thought to have been discounted. However, further studies reproduced the results of FAS I inhibition as the putative mechanism first in whole cell assay of replicating M. tuberculosis bacilli which have shown that pyrazinoic acid and its ester inhibit the synthesis of fatty acids. This study was followed by in vitro assay of tuberculous FAS I enzyme that tested the activity with pyrazinamide, pyrazinoic acid and several classes of pyrazinamide analogs. Pyrazinamide and its analogs inhibited the activity of purified FAS I. It has also been suggested that the accumulation of pyrazinoic acid disrupts membrane potential and interferes with energy production, necessary for survival of M. tuberculosis at an acidic site of infection. Pyrazinoic acid has also been shown to bind to the ribosomal protein S1 (RpsA) and inhibit trans-translation. This may explain the ability of the drug to kill dormant mycobacteria.
Pyrazinamide may be bacteriostatic or bactericidal in action, depending on the concentration of the drug attained at the site of the infection and the susceptibility of the infecting organism. In vitro and in vivo, the drug is active only at a slightly acidic pH. The exact mechanism of action of pyrazinamide has not been fully elucidated. The antimycobacterial activity of pyrazinamide appears to partly depend on conversion of the drug to pyrazinoic acid. Susceptible strains of Mycobacterium tuberculosis produce pyrazinamidase, an enzyme that deaminates pyrazinamide to pyrazinoic acid, and the in vitro susceptibility of a given strain of the organism appears to correspond to its pyrazinamidase activity. In vitro studies indicate that pyrazinoic acid has specific antimycobacterial activity against Mycobacterium tuberculosis. In addition, the fact that pyrazinoic acid lowers the pH of the environment below that which is necessary for growth of Mycobacterium tuberculosis appears to contribute to the drug's antimycobacterial activity in vitro.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 508
Unknown; pyrazinamide may be bacteriostatic or bactericidal, depending on its concentration and the susceptibility of the organism. It is active in vitro at an acidic pH of 5.6 or less, similar to that found in early, active tubercular inflammatory lesions.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2521