
1. Bromide, Pyridostigmine
2. Mestinon
3. Pyridostigmine
1. 101-26-8
2. Mestinon
3. Regonol
4. Kalimin
5. Kalymin
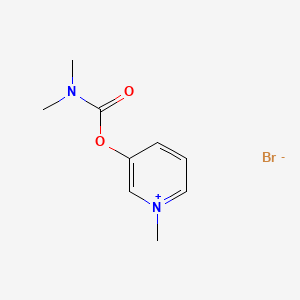
6. 3-(dimethylcarbamoyloxy)-1-methylpyridinium Bromide
7. 3-((dimethylcarbamoyl)oxy)-1-methylpyridin-1-ium Bromide
8. Regonal
9. Mestinon Bromide
10. Pyridostigmini Bromidum
11. Pyridostigmine (bromide)
12. (1-methylpyridin-1-ium-3-yl) N,n-dimethylcarbamate;bromide
13. Nsc-679759
14. Nsc-758435
15. Pyridinium, 3-(((dimethylamino)carbonyl)oxy)-1-methyl-, Bromide
16. Mls000028385
17. 3-[(dimethylcarbamoyl)oxy]-1-methylpyridin-1-ium Bromide
18. Kvi301na53
19. Pyridinium, 3-[[(dimethylamino)carbonyl]oxy]-1-methyl-, Bromide
20. Ro 1-5130
21. 3-hydroxy-1-methylpyridinium Bromide Dimethylcarbamate
22. Ncgc00094324-02
23. 3-(((dimethylamino)carbonyl)oxy)-1-methylpyridinium Bromide
24. Smr000058605
25. Ro-1-5130
26. 3-(dimethylcarbamyloxy)-1-methylpyridinium Bromide
27. Dsstox_cid_3540
28. 1-methyl-3-hydroxypyridinium Bromide Dimethylcarbamate
29. Dsstox_rid_77069
30. Dsstox_gsid_23540
31. Dimethylcarbamic Acid Ester Of 3-hydroxy-1-methylpyridinium Bromide
32. Chembl812
33. Carbamic Acid, Dimethyl-, Ester With 3-hydroxy-1-methylpyridinium Bromide
34. Unii-19qm69hh21
35. 3-dimethylcarbamoyloxy-1-methylpyridinium Bromide;3-dimethylcarbamoyloxy-1-methylpyridinium Bromide
36. Cas-101-26-8
37. Piridostigmina Bromuro [dcit]
38. Ccris 6798
39. Piridostigmina Bromuro
40. Hsdb 3924
41. Sr-01000003072
42. Bromure De Pyridostigmine
43. Bromuro De Piridostigmina
44. Einecs 202-929-9
45. Pyridostigmini Bromidum [inn-latin]
46. Bromure De Pyridostigmine [inn-french]
47. 3-{[(dimethylamino)carbonyl]oxy}-1-methylpyridinium Bromide
48. Unii-kvi301na53
49. Bromuro De Piridostigmina [inn-spanish]
50. Mestinon-sr
51. Pyridinium, 3-[[(dimethylamino)carbonyl]oxy]-1-methyl-, Bromide (1:1)
52. Pyridinium, 3-(((dimethylamino)carbonyl)oxy)-1-methyl-, Bromide (1:1)
53. Mestinon (tn)
54. Pyridostigminebromine
55. Mfcd00079283
56. Pyridinium, 3-hydroxyl-1-methyl-, Bromide, Dimethylcarbamate
57. Opera_id_420
58. 3-hydroxy-1-methylpyridinium Bromide Dimethylcarbamate (ester)
59. Pyridostigmine Bromide [usp:inn:ban:jan]
60. Schembl41147
61. Mls001074080
62. Spectrum1503240
63. Chebi:8666
64. 3-(dimethylaminocarbonyloxy)-1-methylpyridinium Bromide
65. Dtxsid9023540
66. Hms500k09
67. Hy-b0207a
68. Pyridostigmine Bromide (mestinon)
69. Hms1922m05
70. Hms2092p14
71. Hms2234d06
72. Hms3259m16
73. Hms3263o11
74. Hms3369p02
75. Hms3651g13
76. Hms3884m21
77. Pharmakon1600-01503240
78. Pyridostigmine Bromide [mi]
79. Bcp02148
80. Pyridostigmine Bromide [inn]
81. Pyridostigmine Bromide [jan]
82. Tox21_111266
83. Tox21_501035
84. Ccg-40306
85. Nsc679759
86. Nsc758435
87. Pyridinium, 3-hydroxy-1-methyl-, Bromide, Dimethylcarbamate (ester)
88. Pyridostigmine Bromide [hsdb]
89. S1608
90. Akos015895321
91. Pyridostigmine Bromide [mart.]
92. Tox21_111266_1
93. Ac-8143
94. Lp01035
95. Nc00581
96. Nsc 758435
97. Pyridostigmine Bromide [usp-rs]
98. Pyridostigmine Bromide [who-dd]
99. Pyridostigmine Bromide [who-ip]
100. Ncgc00015862-07
101. Ncgc00094324-01
102. Ncgc00094324-03
103. Ncgc00094324-04
104. Ncgc00261720-01
105. Pyridostigmine Bromide (jp17/usp/inn)
106. As-13164
107. Pyridostigmine Bromide [ep Impurity]
108. Pyridostigmine Bromide [orange Book]
109. Eu-0101035
110. Ft-0603307
111. P1339
112. Pyridostigmine Bromide [ep Monograph]
113. Sw199029-2
114. Pyridostigmine Bromide [usp Monograph]
115. Pyridostigmini Bromidum [who-ip Latin]
116. D00487
117. P 9797
118. T71526
119. Q-201644
120. Sr-01000003072-2
121. Sr-01000003072-4
122. Q26840825
123. 3-((dimethylcarbamoyl)oxy)-1-methylpyridin-1-iumbromide
124. Z1541632810
125. Pyridostigmine Bromide, European Pharmacopoeia (ep) Reference Standard
126. Pyridostigmine Bromide, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 261.12 g/mol |
|---|---|
| Molecular Formula | C9H13BrN2O2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 260.01604 g/mol |
| Monoisotopic Mass | 260.01604 g/mol |
| Topological Polar Surface Area | 33.4 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 183 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Mestinon |
| PubMed Health | Pyridostigmine Bromide (By mouth) |
| Drug Classes | Central Nervous System Agent, Immunological Agent, Nerve Gas Antidote |
| Drug Label | Mestinon (pyridostigmine bromide) is an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is:Mestinon is available in the following forms: Syru... |
| Active Ingredient | Pyridostigmine bromide |
| Dosage Form | Tablet, extended release; Tablet; Syrup; Injectable |
| Route | Injection; Oral |
| Strength | 180mg; 60mg/5ml; 5mg/ml; 60mg |
| Market Status | Prescription |
| Company | Valeant Pharm North; Valeant Pharm Intl; Valeant Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Pyridostigmine bromide |
| PubMed Health | Pyridostigmine Bromide (By mouth) |
| Drug Classes | Central Nervous System Agent, Immunological Agent, Nerve Gas Antidote |
| Drug Label | Pyridostigmine bromide is an orally active, reversible cholinesterase inhibitor. Its chemical name is: 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate.CAS registration number is 101-26-8.Pyridostigmine bromide has a molecular formula of C9H13B... |
| Active Ingredient | Pyridostigmine bromide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 60mg |
| Market Status | Prescription |
| Company | Impax Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Mestinon |
| PubMed Health | Pyridostigmine Bromide (By mouth) |
| Drug Classes | Central Nervous System Agent, Immunological Agent, Nerve Gas Antidote |
| Drug Label | Mestinon (pyridostigmine bromide) is an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is:Mestinon is available in the following forms: Syru... |
| Active Ingredient | Pyridostigmine bromide |
| Dosage Form | Tablet, extended release; Tablet; Syrup; Injectable |
| Route | Injection; Oral |
| Strength | 180mg; 60mg/5ml; 5mg/ml; 60mg |
| Market Status | Prescription |
| Company | Valeant Pharm North; Valeant Pharm Intl; Valeant Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Pyridostigmine bromide |
| PubMed Health | Pyridostigmine Bromide (By mouth) |
| Drug Classes | Central Nervous System Agent, Immunological Agent, Nerve Gas Antidote |
| Drug Label | Pyridostigmine bromide is an orally active, reversible cholinesterase inhibitor. Its chemical name is: 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate.CAS registration number is 101-26-8.Pyridostigmine bromide has a molecular formula of C9H13B... |
| Active Ingredient | Pyridostigmine bromide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 60mg |
| Market Status | Prescription |
| Company | Impax Labs |
Cholinesterase Inhibitors; Parasympathomimetics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
A QUATERNARY AMMONIUM ANTICHOLINESTERASE DRUG ...PRINCIPAL USE IS IN THE TREATMENT OF MYASTHENIA GRAVIS. ...
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 842
DOSAGE: THE EQUIVALENT PARENTERAL DOSE OF...PYRIDOSTIGMINE IS APPROX 1/30TH OF THE ORAL DOSE.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 1029
PYRIDOSTIGMINE HAS A SLOWER ONSET (13 MIN) THAN EDROPHONIUM (3 MIN) OR NEOSTIGMINE (6-8 MIN), BUT A LONGER DURATION OF ACTION THAN EITHER. FOR THIS REASON, IT HAS BEEN RECOMMENDED FOR PATIENTS WITH RENAL IMPAIRMENT.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 424
For more Therapeutic Uses (Complete) data for PYRIDOSTIGMINE BROMIDE (9 total), please visit the HSDB record page.
BROMIDE SENSITIVITY OCCASIONALLY OCCURS.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 842
Maternal Medication usually Compatible with Breast-Feeding: Pyridostigmine: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 141 (1994)
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
ITS ONSET OF ACTION BY ORAL ROUTE IS ABOUT 320 MIN...ITS DURATION OF ACTION BY THE ORAL ROUTE IS USUALLY SOMEWHAT LONGER AND ABSORPTION IS LESS ERRATIC THAN NEOSTIGMINE, WHICH ARE ADVANTAGES.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 842
Plasma concn of pyridostigmine was determined in 2 nursing mothers who were receiving oral doses of pyridostigmine bromide, 120-300 mg daily. The drug was not detectable in infant plasma and there were no signs of drug effects in the infant.
Hardell LI et al; Pyridostigmine in human breast milk; Br J Clin Pharmacol 14 (Oct): 565-7 (1982)
PYRIDOSTIGMINE AND ITS QUATERNARY ALCOHOL ARE...THE PREDOMINANT ENTITIES FOUND IN URINE AFTER ADMIN OF THIS DRUG TO MAN.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 108
After admin of pyridostigmine bromide (200 nmol/kg, iv) to human subjects, the disposition half-life was 0.6-1.78 min and terminal half-life was 14.81-37.01 min. Clearance was 9.3-26.5 ml/min/kg which was greater than the presumptive value for glomerular filtration rate and the vol of distribution was 246.5-833.9 ml/kg.
Calvey TN et al; Kinetics of intravenous pyridostigmine in man; Br J Clin Pharmacol 11 (4): 406-8 (1981)
...PHARMACOLOGICAL EFFECTS OF ANTICHOLINESTERASE AGENTS ARE DUE PRIMARILY TO PREVENTION OF HYDROLYSIS OF /ACH/ ACETYLCHOLINE BY ACHE /ACETYLCHOLINESTERASE/ @ SITES OF CHOLINERGIC TRANSMISSION. TRANSMITTER THUS ACCUMULATES, AND THE ACTION OF ACH /ACETYLCHOLINE/ THAT IS LIBERATED BY CHOLINERGIC IMPULSES OR THAT LEAKS FROM THE NERVE ENDING IS ENHANCED.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 103
Following admin of pyridostigmine bromide to rats, erythrocyte acetylcholinesterase activity recovered only slowly due to the covalent nature of inhibition. The logarithm of the plasma concn of pyridostigmine bromide was linearly related to the increase in tibialis twitch tension due to facilitation of neuromuscular transmission.
Barber HE et al; The relationship between the pharmacokinetics, cholinesterase inhibition and facilitation of twitch tension of the quaternary ammonium anticholinesterase drugs, neostigmine, pyridostigmine, edrophonium and 3-hydroxyphenyltrimethylammonium; Br J Pharmacol 66 (4): 525-30 (1979)
Of 12 analogs of pyridostigmine prepared by reacting 2-substituted 3-pyridinols with the desired carbamoyl chloride 2-iodo-3-(dimethylcarbamoyloxy)pyridine methiodide was the most active inhibitor of acetylcholinesterase and butyrylcholinesterase. The progressive inhibition curves for AChE and BuChE are compared and related to ionic attraction and steric requirements of the inhibitors.
Millner OE Jr et al; Synthesis and enzymic evaluation of pyridostigmine analogs used to probe the active sites of acetylcholinesterase and butyrylcholinesterase; J Med Chem 17 (1): 13-8 (1974)