
1. Chloridin
2. Daraprim
3. Malocide
4. Tindurine
1. 58-14-0
2. Daraprim
3. Chloridine
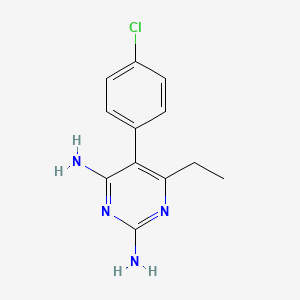
4. 5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine
5. Diaminopyritamin
6. Ethylpyrimidine
7. Pirimetamin
8. Chloridin
9. Chloridyn
10. Pirimecidan
11. Pirimetamina
12. Pyrimethamin
13. Malocide
14. Primethamine
15. Darachlor
16. Erbaprelina
17. Khloridin
18. Tindurin
19. Malocid
20. 5-(4-chlorophenyl)-6-ethyl-2,4-pyrimidinediamine
21. Pyrimethaminum
22. Darapram
23. Daraprime
24. Malacid
25. Maloprim
26. Pyremethamine
27. 2,4-pyrimidinediamine, 5-(4-chlorophenyl)-6-ethyl-
28. 2,4-diamino-5-(p-chlorophenyl)-6-ethylpyrimidine
29. Rp 4753
30. Tcmdc-125860
31. 2,4-diamino-5-chlorophenyl-6-ethylpyrimidine
32. Bw 50-63
33. 2,4-diamino-5-(4-chlorophenyl)-6-ethylpyrimidine
34. 5-(4'-chlorophenyl)-2,4-diamino-6-ethylpyrimidine
35. Nci-c01683
36. Gnf-pf-5586
37. Wr 2978
38. 5-(4-chlorophenyl)-6-ethyl-2,4-diaminopyrimidine
39. Nsc-3061
40. 4753 R.p.
41. Tcmdc-123831
42. Chembl36
43. 5-(4-chloro-phenyl)-6-ethyl-pyrimidine-2,4-diamine
44. 2,4-pyrimidinediamine, 5-(p-chlorophenyl)-6-ethyl-
45. Mls000028606
46. Mls002701881
47. Chebi:8673
48. Z3614qox8w
49. Daraclor
50. Nsc3061
51. 5-(4-chlorophenyl)-6-ethyl-pyrimidine-2,4-diamine
52. Pyrimidine, 2,4-diamino-5-(p-chlorophenyl)-6-ethyl-
53. Rp-4753
54. Wr-2978
55. Cas-58-14-0
56. Ncgc00016256-08
57. Smr000058714
58. Tinduring
59. Pirimetamina [spanish]
60. Dsstox_cid_1217
61. Dsstox_rid_76016
62. Dsstox_gsid_21217
63. Cp6
64. Pirimetamina [inn-spanish]
65. Pyrimethaminum [inn-latin]
66. Brd9204
67. Brd-9204
68. Daraprim (tn)
69. Ccris 546
70. Pyrimethamine (pyr)
71. Sr-01000003150
72. Nsc 3061
73. Einecs 200-364-2
74. Brn 0219864
75. Unii-z3614qox8w
76. Crl-8131 & Pyrimethamine
77. Crl-8142 & Pyrimethamine
78. Lactoferrin B & Pyrimethamine
79. Lactoferrin H & Pyrimethamine
80. Ai3-25005
81. Pyrimethamine (jan/usp/inn)
82. Azt + Pyrimethamine Combination
83. Hsdb 8042
84. Exr-101
85. Pyrimethamine [usp:inn:ban:jan]
86. Prestwick_504
87. Mfcd00057350
88. Bw 5063
89. Daraclor (salt/mix)
90. Spectrum_000906
91. 4km0
92. Cpd000058714
93. Opera_id_1437
94. Prestwick0_000037
95. Prestwick1_000037
96. Prestwick2_000037
97. Prestwick3_000037
98. Spectrum2_000886
99. Spectrum3_001701
100. Spectrum4_000494
101. Spectrum5_001447
102. Pyrimethamine [mi]
103. Pyrimethamine [inn]
104. Pyrimethamine [jan]
105. Cid_4993
106. Nciopen2_008313
107. Pyrimethamine [iarc]
108. Bidd:pxr0173
109. Schembl25129
110. Bspbio_000133
111. Bspbio_003282
112. Kbiogr_001007
113. Kbioss_001386
114. Pyrimethamine [vandf]
115. Mls001148621
116. Mls002454446
117. Bidd:gt0149
118. Divk1c_000652
119. Pyrimethamine [mart.]
120. Spectrum1500520
121. Spbio_000672
122. Spbio_002054
123. Pyrimethamine [usp-rs]
124. Pyrimethamine [who-dd]
125. Pyrimethamine [who-ip]
126. Bpbio1_000147
127. Gtpl4800
128. Dtxsid9021217
129. Bdbm18512
130. Hms502a14
131. Kbio1_000652
132. Kbio2_001386
133. Kbio2_003954
134. Kbio2_006522
135. Kbio3_002502
136. Zinc57464
137. Ninds_000652
138. Pirimecidan;pirimetamin;rp 4753
139. Hms1568g15
140. Hms1920n12
141. Hms2092e13
142. Hms2095g15
143. Hms2235a17
144. Hms3259c04
145. Hms3371l07
146. Hms3655d09
147. Hms3675p11
148. Hms3712g15
149. Hms3743o05
150. Hms3871i03
151. Pharmakon1600-01500520
152. Pyrimethamine [green Book]
153. Pyrimethamine [orange Book]
154. 2, 5-(p-chlorophenyl)-6-ethyl-
155. 2, 5-(4-chlorophenyl)-6-ethyl-
156. Pyrimethamine [ep Monograph]
157. Tox21_110332
158. Tox21_201834
159. Tox21_300129
160. Ccg-39626
161. Nsc757306
162. Pyrimethamine [usp Monograph]
163. Pyrimethaminum [who-ip Latin]
164. Akos015892534
165. Fansidar Component Pyrimethamine
166. Tox21_110332_1
167. Ab02313
168. Ac-7879
169. Cs-1717
170. Db00205
171. Ks-5223
172. Nc00528
173. Nsc-757306
174. Idi1_000652
175. Ncgc00016256-01
176. Ncgc00016256-02
177. Ncgc00016256-03
178. Ncgc00016256-04
179. Ncgc00016256-05
180. Ncgc00016256-06
181. Ncgc00016256-07
182. Ncgc00016256-09
183. Ncgc00016256-10
184. Ncgc00016256-11
185. Ncgc00016256-12
186. Ncgc00016256-13
187. Ncgc00016256-14
188. Ncgc00016256-16
189. Ncgc00016256-17
190. Ncgc00023188-03
191. Ncgc00023188-04
192. Ncgc00023188-05
193. Ncgc00023188-06
194. Ncgc00023188-07
195. Ncgc00254199-01
196. Ncgc00259383-01
197. Wln: T6n Cnj Bz Dz Er Dg& F2
198. Hy-18062
199. Nci60_002604
200. Pyrimethamine Component Of Fansidar
201. Sbi-0051500.p003
202. Db-053158
203. Ab00052084
204. Ft-0631253
205. P2037
206. S2006
207. Sw196698-3
208. C07391
209. D00488
210. Mls-0002822.0001
211. Ab00052084-21
212. Ab00052084_22
213. Ab00052084_25
214. 057p350
215. 2,4-diamino-5(4-chlorophenyl)-6-ethylpyrimidine
216. A831755
217. L000713
218. Pyrimethamine, Vetranal(tm), Analytical Standard
219. Pyrimidine,4-diamino-5-(p-chlorophenyl)-6-ethyl-
220. Q421072
221. 2,4,-diamino-5-(4-chlorophenyl)-6-ethylpyrimidine
222. 2,4-diamino-5-(4-chloro-phenyl)-6-ethylpyrimidine
223. 2,4-diamino-6-ethyl-5-(4-chlorophenyl)pyrimidine
224. Q-201648
225. Sr-01000003150-2
226. Sr-01000003150-4
227. Brd-k88429204-001-05-4
228. Brd-k88429204-001-18-7
229. Brd-k88429204-001-21-1
230. Brd-k88429204-001-36-9
231. Z2065727768
232. 2,4-diamino-5-(4-chlorophenyl)-6-ethylpyrimidine, 97%
233. Pyrimethamine, European Pharmacopoeia (ep) Reference Standard
234. Pyrimethamine, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 248.71 g/mol |
|---|---|
| Molecular Formula | C12H13ClN4 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 248.0828741 g/mol |
| Monoisotopic Mass | 248.0828741 g/mol |
| Topological Polar Surface Area | 77.8 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 243 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Daraprim |
| PubMed Health | Pyrimethamine (By mouth) |
| Drug Classes | Antimalarial |
| Drug Label | DARAPRIM (pyrimethamine) is an antiparasitic compound available in tablet form for oral administration. Each scored tablet contains 25mg pyrimethamine and the inactive ingredients corn and potato starch, lactose, and magnesium stearate. Pyrimethami... |
| Active Ingredient | Pyrimethamine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Amedra Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Daraprim |
| PubMed Health | Pyrimethamine (By mouth) |
| Drug Classes | Antimalarial |
| Drug Label | DARAPRIM (pyrimethamine) is an antiparasitic compound available in tablet form for oral administration. Each scored tablet contains 25mg pyrimethamine and the inactive ingredients corn and potato starch, lactose, and magnesium stearate. Pyrimethami... |
| Active Ingredient | Pyrimethamine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Amedra Pharms |
Although pyrimethamine has been used alone for suppression or chemoprophylaxis of malaria in travelers, the drug is no longer recommended by the US Centers for Disease Control and Prevention (CDC) or other experts for prevention of malaria. The manufacturer states that pyrimethamine should only be used for suppression or chemoprophylaxis of malaria caused by Plasmodium known to be susceptible to the drug. However, resistance to pyrimethamine is prevalent worldwide and the drug alone is not a suitable chemoprophylaxis regimen for travelers to most areas of the world. /Included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Pyrimethamine is used in conjunction with sulfadiazine or, alternatively, clindamycin, atovaquone, or azithromycin for the treatment of toxoplasmosis caused by Toxoplasma gondii. /Included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Oral or parenteral leucovorin is used with pyrimethamine in these regimens to prevent pyrimethamine-induced adverse hematologic effects. /Included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Although co-trimoxazole generally is considered the drug of choice for the treatment of GI infections caused by Isospora belli, pyrimethamine has been used for the treatment of isosporiasis in some patients (e.g., HIV-infected patients) when co-trimoxazole was contraindicated, including those with sulfonamide sensitivity. /NOT included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
For more Therapeutic Uses (Complete) data for Pyrimethamine (16 total), please visit the HSDB record page.
High dosages of pyrimethamine may result in adverse nervous system effects including ataxia, tremors, seizures, and respiratory failure. Headache, light-headedness, insomnia, depression, malaise, fatigue, and irritability have been reported rarely with pyrimethamine. Reversible hyperesthesia has been reported rarely with sulfadoxine and pyrimethamine. Other adverse nervous system effects reported with sulfonamides or pyrimethamine include peripheral neuritis, hallucinations, tinnitus, vertigo, muscle weakness, nervousness, and polyneuritis.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Sensitivity reactions, occasionally severe (e.g., Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme, anaphylaxis) have been reported with pyrimethamine, especially when the drug was used with a sulfonamide. Severe, sometimes fatal, hypersensitivity reactions have occurred with the fixed-combination preparation of sulfadoxine and pyrimethamine. In most reported cases, fatalities resulted from severe cutaneous reactions, including erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Pulmonary hypersensitivity reactions and a fatal reaction involving the skin, liver, and kidneys also have been reported. Fatal hepatitis also has been reported with the fixed-combination drug.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Severe reactions to sulfadoxine and pyrimethamine have occurred in travelers who received 2-9 doses of the drug for prophylaxis of malaria, but have not been reported to date following a single dose of the drug such as that used in the treatment of malaria. It is estimated that the incidence of severe cutaneous adverse reactions ranges from 1/8000 to 1/5000 and that the incidence of fatal cutaneous reactions ranges from 1/25,000 to 1/11,000 in US travelers receiving chemoprophylaxis with sulfadoxine and pyrimethamine.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Anorexia, abdominal cramps, diarrhea, and vomiting may occur with high dosages of pyrimethamine. Anorexia and vomiting may be minimized by reducing dosage of pyrimethamine or by administering the drug with meals. Atrophic glossitis or gastritis also has been reported with high dosages of pyrimethamine. Other adverse GI effects reported with sulfonamides or with pyrimethamine include stomatitis, nausea, abdominal pain, and feeling of fullness.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
For more Drug Warnings (Complete) data for Pyrimethamine (21 total), please visit the HSDB record page.
The concentration of chloroquine, dapsone and pyrimethamine in plasma and milk were measured following the coadministration of a single dose of chloroquine and Maloprim to lactating women. The milk to plasma area under the concentration-time curve (AUC) ratio ranged from 1.96 to 4.26 for chloroquine, 0.22 to 0.45 for dapsone and 0.46 to 0.66 for pyrimethamine. Assuming a daily milk ingestion of 1 l by the infant, the maximum percentage of the maternal dose for chloroquine, dapsone and pyrimethamine in milk was 4.2%, 14.3% and 45.6%, respectively, over a 9 day period.
PMID:3567020 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1401222 Edstein MD et al; Br J Clin Pharmacol 22 (6): 733-5 (1986)
The fatal dose is variable, with the smallest reported fatal single dose being 375 mg.
US Natl Inst Health; DailyMed. Current Medication Information for DARAPRIM (pyrimethamine) tablet (October 2010). Available from, as of June 26, 2012: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=DARAPRIM
For the treatment of toxoplasmosis and acute malaria; For the prevention of malaria in areas non-resistant to pyrimethamine
FDA Label
Pyrimethamine is an antiparasitic compound commonly used as an adjunct in the treatment of uncomplicated, chloroquine resistant, P. falciparum malaria. Pyrimethamine is a folic acid antagonist and the rationale for its therapeutic action is based on the differential requirement between host and parasite for nucleic acid precursors involved in growth. This activity is highly selective against plasmodia and Toxoplasma gondii. Pyrimethamine possesses blood schizonticidal and some tissue schizonticidal activity against malaria parasites of humans. However, the 4-amino-quinoline compounds are more effective against the erythrocytic schizonts. It does not destroy gametocytes, but arrests sporogony in the mosquito. The action of pyrimethamine against Toxoplasma gondii is greatly enhanced when used in conjunction with sulfonamides.
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
Folic Acid Antagonists
Inhibitors of the enzyme, dihydrofolate reductase (TETRAHYDROFOLATE DEHYDROGENASE), which converts dihydrofolate (FH2) to tetrahydrofolate (FH4). They are frequently used in cancer chemotherapy. (From AMA, Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Folic Acid Antagonists.)
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
P01BD01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01B - Antimalarials
P01BD - Diaminopyrimidines
P01BD01 - Pyrimethamine
Absorption
Well absorbed with peak levels occurring between 2 to 6 hours following administration
The concentration of chloroquine, dapsone and pyrimethamine in plasma and milk were measured following the coadministration of a single dose of chloroquine and Maloprim to lactating women. The milk to plasma area under the concentration-time curve (AUC) ratio ranged from 1.96 to 4.26 for chloroquine, 0.22 to 0.45 for dapsone and 0.46 to 0.66 for pyrimethamine. Assuming a daily milk ingestion of 1 l by the infant, the maximum percentage of the maternal dose for chloroquine, dapsone and pyrimethamine in milk was 4.2%, 14.3% and 45.6%, respectively, over a 9 day period.
PMID:3567020 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1401222 Edstein MD et al; Br J Clin Pharmacol 22 (6): 733-5 (1986)
Pyrimethamine is excreted into milk. It is estimated that approximately 3-4 mg of the drug would be ingested by a nursing infant over the first 48-hour period following administration of a single 75-mg oral dose to the mother.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
In this study, the kinetics of pyrimethamine elimination via the urine was investigated. The experiments were carried out on six healthy male volunteers aged 23-32 years. The drug was administered orally (p.o.) in a single dose at three different concentrations i.e.: 50, 75 and 100 mg. The concentration of the drug in the urine was determined via the modified method of Bonini et al. and Garber et al. It was found that 13.4 +/- 1.3% of the dose eliminated via the urine was in unchanged form. The process of pyrimethamine elimination may be described according to an open kinetic two-compartmental model: the formula showing the course of pyrimethamine elimination over time has been given. Several examples of the quantitative exposure test have been proposed, which allow the calculation of the drug dose absorbed and thus the degree of toxicity to be determined. This test can also be useful in a controlled clinical setting.
PMID:20166434 Mouankie JB et al; Eur J Drug Metab Pharmacokinet 34 (3-4): 169-72 (2009)
A pharmacokinetic study of pyrimethamine was carried out in 4- (103-115 g) and 12-week-old (260-280 g) white male Wistar rats fed a standard diet containing 24% protein, and a low-protein diet containing 8% protein. After intragastric administration of the drug in a single dose of 40 mg/kg body weight, the concentrations of pyrimethamine in the blood were determined at different time points from 15 min to 20 hours post-dose. On the basis of the results obtained, a number of parameters characterizing the course of absorption and elimination of the drug from the blood were calculated. The majority of parameters were dependent on both age and type of diet. The greatest bioavailability was observed in the 4-week-old rats: for the animals fed the low-protein diet, the area under the concentration-time curve (AUC) amounted to 593.0 and for those on the standard diet the AUC was 503.1. In the older rats, this parameter was 339.3 and 228.1 respectively. The k(e) values were lower in the younger rats (i.e. 0.0121 hr(-1) and 0.0135 h(-1)) than in the older animals (i.e. 0.0164 h(-1) and 0.0193 hr(-1) respectively). The elimination half-life (t1/2) was higher in the 4-week-old rats (i.e. 57.1 hr; 8% protein, and 51.2 hr; 24% protein) than in the 12-week-old animals (i.e. 42.4 hr; 8% protein, and 36.0 hr; 24% protein).
PMID:20166435 Mouankie JB et al; Eur J Drug Metab Pharmacokinet 34 (3-4): 173-6 (2009)
For more Absorption, Distribution and Excretion (Complete) data for Pyrimethamine (10 total), please visit the HSDB record page.
Hepatic
Pyrimethamine is metabolized to several unidentified metabolites. About 5% of a dose of sulfadoxine is present in plasma as an acetylated metabolite and about 2-3% is present as the glucuronide.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
96 hours
Pyrimethamine reportedly has an average plasma half-life of 111 hours (range: 54-148 hours). The plasma half-life of sulfadoxine reportedly averages 169 hours (range: 100-231 hours).
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
To determine pyrimethamine levels in sera, cerebrospinal fluid, and ventricular fluid in infants, specimens were examined from 37 infants, ages 10 days to 1.5 yr, receiving pyrimethamine 1 mg/kg of body weight daily for 2 months followed by the same dosage each Monday, Wednesday, and Friday for treatment of suspect or proven congenital toxoplasmosis. The pyrimethamine half-life obtained from serum of 9 babies was 64 hr, which was significantly different than for 2 infants taking phenobarbital (33 hr).
McLeod R et al; Antimicrob Agents Chemother 36 (May): 1040-8 (1992)
A pharmacokinetic study of pyrimethamine was carried out in 4- (103-115 g) and 12-week-old (260-280 g) white male Wistar rats fed a standard diet containing 24% protein, and a low-protein diet containing 8% protein. After intragastric administration of the drug in a single dose of 40 mg/kg body weight, the concentrations of pyrimethamine in the blood were determined at different time points from 15 min to 20 hours post-dose...The elimination half-life (t1/2) was higher in the 4-week-old rats (i.e. 57.1 hr; 8% protein, and 51.2 hr; 24% protein) than in the 12-week-old animals (i.e. 42.4 hr; 8% protein, and 36.0 hr; 24% protein).
PMID:20166435 Mouankie JB et al; Eur J Drug Metab Pharmacokinet 34 (3-4): 173-6 (2009)
Pyrimethamine inhibits the dihydrofolate reductase of plasmodia and thereby blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication. This leads to failure of nuclear division at the time of schizont formation in erythrocytes and liver.
Pyrimethamine is an antimalarial drug that has also been used successfully to treat autoimmune diseases such as lymphoproliferative syndrome. In this work, the effect of pyrimethamine (PYR) on the production of free radicals in malaria-infected mice was studied to better understand the drug's immunomodulatory properties. BALB/c and CBA/Ca mice were infected with Plasmodium yoelii 17XL. Seven days after infection, mice were treated with PYR or vehicle and sacrificed 24h later. Treatment with PYR increased superoxide dismutase and glutathione peroxidase activities in erythrocytes and the liver, augmented the levels of nitric oxide in the serum, and upregulated mRNA levels of superoxide dismutase, glutathione peroxidase, catalase, and iNOS in the spleen. In addition, PYR increased lipoperoxidation and protein carbonylation in infected mice. Our results indicate that P. yoelii 17XL reduces oxidative stress in infected cells, while PYR induces it, which is associated with increased parasite elimination. Thus, it is possible that oxidative stress generated by pyrimethamine is also involved in its immunomodulatory mechanism of action.
PMID:20193682 Legorreta-Herrera M et al; Exp Parasitol 126 (3): 381-8 (2010)
Co-infection of human immunodeficiency virus (HIV) with malaria is one of the pandemic problems in Africa and parts of Asia. Here we investigated the impact of pyrimethamine (PYR) and two other clinical anti-malarial drugs (chloroquine [CQ] or artemisinin [ART]) on HIV-1 replication. Peripheral blood mononuclear cells (PBMCs) or MT-2 cells were infected with HIV(NL4.3) strain and treated with different concentrations of the anti-malarial drugs. HIV-1 replication was measured using p24 ELISA. We show that 10 uM CQ and ART inhibited HIV-1 replication by 76% and 60% in PBMCs, respectively, but not in MT-2 cells. In contrast, 10 uM PYR enhanced HIV-1 replication in MT-2 cells by >10-fold. A series of molecular mechanism studies revealed that PYR increased intracellular HIV gag proteins without affecting the promoter or the reverse transcriptase activity. The effect of PYR was independent of HTLV-1 produced by MT-2 cells. Of interest, PYR treatment led to S-phase accumulation and increased AZT and d4T antiviral activity by ~ 4-fold. Taken together, we show that PYR significantly enhances HIV-1 replication by affecting the cellular machinery. Our results could be relevant for the management of malaria and HIV particularly in regions where HIV-1 and malaria epidemics overlap.
PMID:20800626 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2956596 Oguariri RM et al; Virus Res 153 (2): 269-76 (2010)
Autosomal dominant polycystic kidney disease (ADPKD) is a commonly inherited disorder mostly caused by mutations in PKD1, encoding polycystin-1 (PC1). The disease is characterized by development and growth of epithelium-lined cyst in both kidneys, often leading to renal failure. There is no specific treatment for this disease. Here, we report a sustained activation of the transcription factor signal transducer and activator of transcription 3 (STAT3) in ischemic injured and uninjured Pkd1 knockout polycystic kidneys and in human ADPKD kidneys. Through a chemical library screen, we identified the anti-parasitic compound pyrimethamine as an inhibitor of STAT3 function. Treatment with pyrimethamine decreases cell proliferation in human ADPKD cells and blocks renal cyst formation in an adult and a neonatal PKD mouse model. Moreover, we demonstrated that a specific STAT3 inhibitor, S3I-201, reduces cyst formation and growth in a neonatal PKD mouse model. Our results suggest that PC1 acts as a negative regulator of STAT3 and that blocking STAT3 signaling with pyrimethamine or similar drugs may be an attractive therapy for human ADPKD.
PMID:21821671 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3188991 Takakura A et al; Hum Mol Genet 20 (21): 4143-54 (2011)
The unresponsiveness of metastatic melanoma to conventional chemotherapeutic and biological agents is largely due to the development of resistance to apoptosis. Pyrimethamine belongs to the group of antifolate drugs, and in addition to antiprotozoan effects, it exerts a strong proapoptotic activity, which we recently characterized in human T lymphocytes. However, no data regarding pyrimethamine anticancer activity are available thus far. To this end, we examined the in vitro effects of pyrimethamine on apoptosis, cell cycle distribution, and cell proliferation of human metastatic melanoma cell lines. The in vivo antitumor potential of pyrimethamine was evaluated in a severe combined immunodeficiency (SCID) mouse xenotransplantation model. Our data indicate that pyrimethamine, when used at a clinically relevant concentration, induced apoptosis in metastatic melanoma cells via the activation of the cathepsin B and the caspase cascade (i.e., caspase-8 and caspase-9) and subsequent mitochondrial depolarization. This occurred independently from CD95/Fas engagement. Moreover, pyrimethamine induced a marked inhibition of cell growth and an S-phase cell cycle arrest. Results obtained in SCID mice, injected s.c. with metastatic melanoma cells and treated with pyrimethamine, indicated a significant inhibitory effect on tumor growth. In conclusion, our results suggest that pyrimethamine-induced apoptosis may be considered as a multifaceted process, in which different inducers or regulators of apoptosis are simultaneously implicated, thus permitting death defects of melanoma cells to be bypassed or overcome. On these bases, we hypothesize that pyrimethamine could represent an interesting candidate for the treatment of metastatic melanoma.
PMID:18593930 Giammarioli AM et al; Cancer Res 68 (13): 5291-300 (2008)
Pyrimethamine is a folic acid antagonist and has a mechanism of action similar to that of trimethoprim. By binding to and reversibly inhibiting dihydrofolate reductase, pyrimethamine inhibits the reduction of dihydrofolic acid to tetrahydrofolic acid (folinic acid). Pyrimethamine interferes with the synthesis of tetrahydrofolic acid in malarial parasites at a point immediately succeeding that where sulfonamides act. Sulfadoxine, like other sulfonamides, is a structural analog of p-aminobenzoic acid (PABA) and competitively inhibits dihydrofolic acid synthesis which is necessary for the conversion of PABA to folic acid. The combination of sulfadoxine and pyrimethamine results in a synergistic action against susceptible plasmodia.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012