


1. Ab680
1. Ab-680
2. 2105904-82-1
3. Ab680
4. Quemliclustat [usan]
5. J6k8wsv73a
6. Chembl4471306
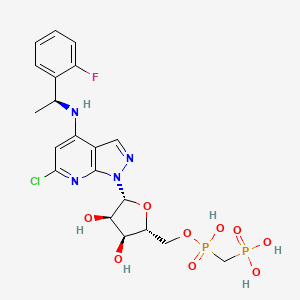
7. [[(2~{r},3~{s},4~{r},5~{r})-5-[6-chloranyl-4-[[(1~{s})-1-(2-fluorophenyl)ethyl]amino]pyrazolo[3,4-b]pyridin-1-yl]-3,4-bis(oxidanyl)oxolan-2-yl]methoxy-oxidanyl-phosphoryl]methylphosphonic Acid
8. [[(2r,3s,4r,5r)-5-[6-chloro-4-[[(1s)-1-(2-fluorophenyl)ethyl]amino]pyrazolo[3,4-b]pyridin-1-yl]-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]methylphosphonic Acid
9. Unii-j6k8wsv73a
10. Quemliclustat [inn]
11. Cd73 Inhibitor Ab-680
12. Schembl19100484
13. Gtpl10707
14. Ex-a4998
15. Bdbm50527134
16. Who 11621
17. Ac-36768
18. Ba178946
19. Hy-125286
20. Cs-0090231
21. F78354
22. (((((2r,3s,4r,5r)-5-(6-chloro-4-(((s)-1-(2-fluorophenyl)ethyl)amino)-1h-pyrazolo[3,4-b]pyridin-1-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(hydroxy)phosphoryl)methyl)phosphonic Acid
23. (((2r,3s,4r,5r)-5-(6-chloro-4-((s)-1-(2-fluorophenyl)ethylamino)-1h-pyrazolo[3,4-b]pyridin-1-yl)-3,4-dihydroxytetrahydrofuran-2-ylmethoxy)(hydroxy)phosphorylmethyl)phosphonic Acid
24. 1h-pyrazolo[3,4-b]pyridin-4-amine, 6-chloro-n-[(1s)-1-(2-fluorophenyl)ethyl]-1-[5-o-[hydroxy(phosphonomethyl)phosphinyl]-beta-d-ribofuranosyl]-
25. 2-chloro-n6 -[(1s)-1-(2-fluorophenyl)ethyl]-8-aza-1,7- Dicarbaadenosine 5'- (trihydrogen 2-carbadiphosphate)
26. Qdh
| Molecular Weight | 580.8 g/mol |
|---|---|
| Molecular Formula | C20H24ClFN4O9P2 |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 9 |
| Exact Mass | 580.0691082 g/mol |
| Monoisotopic Mass | 580.0691082 g/mol |
| Topological Polar Surface Area | 197 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 893 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Immune Checkpoint Inhibitors
Drugs that block negative regulator IMMUNE CHECKPOINT proteins (e.g., PD-1 RECEPTOR and CTLA-4 ANTIGEN) thereby increasing suppressed immune activation in immunotherapies. (See all compounds classified as Immune Checkpoint Inhibitors.)