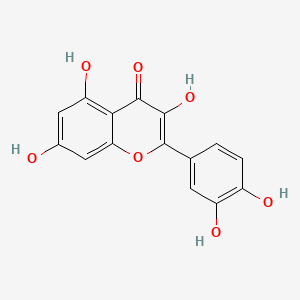

1. 3,3',4',5,7-pentahydroxyflavone
2. Dikvertin
1. 117-39-5
2. Meletin
3. Sophoretin
4. Quercetine
5. Xanthaurine
6. Quercetol
7. Quertine
8. 3,3',4',5,7-pentahydroxyflavone
9. Quercitin
10. 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4h-chromen-4-one
11. Cyanidelonon 1522
12. 3,5,7,3',4'-pentahydroxyflavone
13. Flavin Meletin
14. Quertin
15. T-gelb Bzw. Grun 1
16. C.i. Natural Yellow 10
17. Quercetin Content
18. Kvercetin
19. C.i. 75670
20. 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4h-1-benzopyran-4-one
21. C.i. Natural Red 1
22. 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one
23. Corvitin
24. Cyanidenolon 1522
25. 4h-1-benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-
26. 3',4',5,7-tetrahydroxyflavan-3-ol
27. Flavone, 3,3',4',5,7-pentahydroxy-
28. C.i. Natural Yellow 10 & 13
29. Nsc 9219
30. Lipoflavon
31. Ccris 1639
32. Korvitin
33. Hsdb 3529
34. Nci-c60106
35. 3',4',5,7-tetrahydroxyflavon-3-ol
36. 3'-hydroxykaempferol
37. 3,5,7-trihydroxy-2-(3,4-dihydroxyphenyl)-4h-chromen-4-on
38. Chebi:16243
39. Ai3-26018
40. Nsc9219
41. 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromen-4-one
42. Nsc-9219
43. Chembl50
44. Mfcd00006828
45. 9ikm0i5t1e
46. 2-(3,4-dihydroxy-phenyl)-3,5,7-trihydroxy-chromen-4-one
47. Ci-75670
48. Nsc-57655
49. Kvercetin [czech]
50. 74893-81-5
51. Ldn-0052529
52. Natural Yellow 10
53. Flavone, 3,4',5,5',7-pentahydroxy-
54. 3,5,7-trihydroxy-2-(3,4-dihydroxyphenyl)-4h-chromen-4-one
55. Dsstox_cid_1218
56. Ci Natural Yellow 10
57. 7255-55-2
58. Dsstox_rid_76017
59. Dsstox_gsid_21218
60. Que
61. Brd9794
62. Brd-9794
63. Cas-117-39-5
64. Nsc57655
65. Nsc58588
66. Sr-01000076098
67. Einecs 204-187-1
68. Unii-9ikm0i5t1e
69. Mixcom3_000183
70. Brn 0317313
71. Ci 75670
72. Ritacetin
73. Quer
74. 4dfu
75. 4mra
76. Quercetin2h2o
77. Meletin;sophoretin
78. Kuc104418n
79. Kuc107684n
80. 3,3',4,5,7-pentahydroxyflavone
81. Quercetin-[d3]
82. Lim-5662
83. Lns-5662
84. Tnp00070
85. Tnp00089
86. Ksc-23-76
87. Quercetin_sathishkumar
88. Ksc-10-126
89. Quercetin (sophoretin)
90. Quercetin - Sophoretin
91. Spectrum_000124
92. Tocris-1125
93. 3cf8
94. Quercetin [dsc]
95. Quercetin [mi]
96. Biomolki_000062
97. Quercetin [hsdb]
98. Quercetin [iarc]
99. Quercetin [inci]
100. Maybridge1_008992
101. Prestwick0_000507
102. Prestwick1_000507
103. Prestwick2_000507
104. Prestwick3_000507
105. Spectrum2_000059
106. Spectrum3_000642
107. Spectrum4_000807
108. Spectrum5_001389
109. Lopac-q-0125
110. Quercetin [vandf]
111. P0042
112. C.i. Natural Yellow 13
113. Biomolki2_000068
114. Enicostemma Littorale Blume
115. Upcmld-dp081
116. Q 0125
117. Quercetin [usp-rs]
118. Quercetin [who-dd]
119. Nciopen2_007628
120. Nciopen2_007882
121. Bidd:pxr0007
122. Lopac0_000999
123. Schembl19723
124. Bspbio_000433
125. Bspbio_001068
126. Bspbio_002243
127. Kbiogr_000408
128. Kbiogr_001293
129. Kbioss_000408
130. Kbioss_000584
131. 5-18-05-00494 (beilstein Handbook Reference)
132. Mls006011766
133. Bidd:er0315
134. Divk1c_000485
135. Schembl219729
136. Spectrum1500672
137. Cu-01000012502-3
138. Spbio_000217
139. Spbio_002354
140. Bdbm7460
141. Bpbio1_000477
142. Gtpl5346
143. Megxp0_000381
144. Sgcut00001
145. 3,4',5,7-pentahydroxyflavone
146. Dtxsid4021218
147. Niosh/lk8760000
148. Upcmld-dp081:001
149. Acon1_000560
150. Hms501i07
151. Kbio1_000485
152. Kbio2_000408
153. Kbio2_000584
154. Kbio2_002976
155. Kbio2_003152
156. Kbio2_005544
157. Kbio2_005720
158. Kbio3_000775
159. Kbio3_000776
160. Kbio3_001463
161. 3,7,3',4'-pentahydroxyflavone
162. Ninds_000485
163. 3',5,7-tetrahydroxyflavan-3-ol
164. Bio1_000369
165. Bio1_000858
166. Bio1_001347
167. Bio2_000374
168. Bio2_000854
169. Hms1362f09
170. Hms1792f09
171. Hms1923o19
172. Hms1990f09
173. Hms3263g19
174. Hms3267m12
175. Hms3414j21
176. Hms3649d04
177. Hms3656c15
178. Hms3678j19
179. To_000078
180. Zinc3869685
181. 3,5,7,3',4'-pentahydroxyflavon
182. Tox21_202308
183. Tox21_300285
184. Tox21_500999
185. Bbl005513
186. Ccg-40054
187. Flavone,3',4',5,7-pentahydroxy-
188. Lmpk12110004
189. Nsc 57655
190. Nsc324608
191. Nsc756660
192. S2391
193. Stk365650
194. Quercetin, >=95% (hplc), Solid
195. 3,4',5,5',7-pentahydroxy-flavone
196. Akos000511724
197. Quercetin 1000 Microg/ml In Acetone
198. Cs-3981
199. Db04216
200. Ds-3416
201. Lp00999
202. Nsc-756660
203. Sdccgsbi-0050972.p003
204. Idi1_000485
205. Idi1_002129
206. Ldn 0052529
207. Smp1_000252
208. Ncgc00015870-01
209. Ncgc00015870-02
210. Ncgc00015870-03
211. Ncgc00015870-04
212. Ncgc00015870-05
213. Ncgc00015870-06
214. Ncgc00015870-07
215. Ncgc00015870-08
216. Ncgc00015870-09
217. Ncgc00015870-10
218. Ncgc00015870-11
219. Ncgc00015870-12
220. Ncgc00015870-13
221. Ncgc00015870-14
222. Ncgc00015870-15
223. Ncgc00015870-16
224. Ncgc00015870-17
225. Ncgc00015870-18
226. Ncgc00015870-19
227. Ncgc00015870-21
228. Ncgc00015870-22
229. Ncgc00015870-23
230. Ncgc00015870-24
231. Ncgc00015870-25
232. Ncgc00015870-28
233. Ncgc00015870-48
234. Ncgc00015870-50
235. Ncgc00025016-01
236. Ncgc00025016-02
237. Ncgc00025016-03
238. Ncgc00025016-04
239. Ncgc00025016-05
240. Ncgc00025016-06
241. Ncgc00025016-07
242. Ncgc00025016-08
243. Ncgc00168962-01
244. Ncgc00168962-02
245. Ncgc00168962-03
246. Ncgc00168962-04
247. Ncgc00254218-01
248. Ncgc00259857-01
249. Ncgc00261684-01
250. Quercetin 100 Microg/ml In Acetonitrile
251. Ac-19596
252. Ac-29756
253. Hy-18085
254. Nci60_042036
255. Smr000112559
256. Sy057722
257. (+)-3,3',4',5,7-pentahydroxyflavone
258. Quercetin, Sophoretin, Meletin, Quercetine
259. Eu-0100999
260. Ft-0603318
261. Ft-0655108
262. Lk87600000
263. N1841
264. Q0025
265. Sw148203-4
266. Quercetin; 3,3',4',5,7-pentahydroxyflavone
267. 17q395
268. C00389
269. K00029
270. S00057
271. Quercetin (constituent Of Ginkgo) [dsc]
272. Wln: T66 Bo Evj Cr Cq Dq & Dq Gq Iq
273. Flavone, 3,3',4',5,7-pentahydroxy-, (+)-
274. Q409478
275. Q-200333
276. Sr-01000076098-1
277. Sr-01000076098-3
278. Sr-01000076098-7
279. Sr-01000076098-8
280. Brd-k97399794-001-02-1
281. Brd-k97399794-001-07-0
282. Brd-k97399794-001-09-6
283. Brd-k97399794-001-11-2
284. Brd-k97399794-335-03-1
285. Sr-01000076098-11
286. 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromone;hydrate
287. 49643640-fd4c-4b93-bd28-0d7c2021cc52
288. 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4h-chromen-4-one #
289. Quercetin (constituent Of Hawthorn Leaf With Flower) [dsc]
290. (+)-4h-1-benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-
291. 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4h-benzopyran-4-one
292. 4h-1-benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-,zirconium(2+)salt(1:1)
293. 4h-1-benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-, Zirconium(2+) Salt (1:1)
| Molecular Weight | 302.23 g/mol |
|---|---|
| Molecular Formula | C15H10O7 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | 302.04265265 g/mol |
| Monoisotopic Mass | 302.04265265 g/mol |
| Topological Polar Surface Area | 127 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 488 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Quercetin has been used in medicine to decrease capillary fragility.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V73 P498 (1999)
/EXPL THER/ ... In a randomized, double-blind, placebo-controlled trial ... /among patients with category III chronic prostatitis syndromes (nonbacterial chronic prostatitis and prostatodynia)/ ... Significant improvement was achieved in the treated group, as measured by the NIH chronic prostatitis score. Some 67% of the treated subjects had at least 25% improvement in symptoms, compared with 20% of the placebo group achieving this same level of improvement. In a follow up, unblind, open-label study ... quercetin was combined with bromelain and papain, which may enhance its absorption. In this study, 82% achieved a minimum 25% improvement score.
Thomson Healthcare. PDR for Nutritional Supplements. Thomson Health Care Inc. Montvale, NJ. p.393 (2001)
/EXPL THER/ Lymphocyte protein kinase phosphorylation was inhibited by quercetin in 9 of 11 cancer patients in a phase I clinical trial. Fifty-one patients with microscopically confirmed cancer not amenable to standard therapies and with a life expectancy of at least 12 wk participated in this trial ... The patients were treated at 3-wk intervals at the beginning of the study. Quercetin was admin iv as quercetin dihydrate ... The max allowed dose was reached when 2 of 3 patients on each dose schedule reached grade 3 or 4 general toxicity, or grad 2 renal toxicity, cardiac toxicity, or neurotoxicity. Phosphorylation was inhibited at 1 hr and persisted for 16 hr. In one patient with ovarian cancer refractory to cisplatin, cancer antigen-125 (CA 125) fell from 295 to 55 units/mL after treatment with 2 courses of quercetin ... A hepatoma patient had serum alpha-fetoprotein fall.
Thomson Healthcare. PDR for Herbal Medicines 4th Ed. Thomson Health Care Inc. Montvale, NJ. 2008, p. 1002
/EXPL THER/ ... Quercetin was reported to inhibit tumor necrosis factor-alpha (TNF-alpha) overproduction and attenuate pathophysiological conditions during acute and chronic inflammation ... In asthma, the activation of mast cells and basophils by allergen releases chemical mediators and synthesizeds cytokines leading to inflammatory conditions ... Quercetin was reported to inhibit cytokine expression and synthesis by human basophils ... A metabolite of quercetin, 3-O-methylquercetin (3-MQ), was reported to provide beneficial effects on asthma by inhibiting cAMP- and cGMP-phosphodiesterase (PDE). ...
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 580 (2005)
Although quercetin seems to have potential as an anticancer agent, future studies are needed, because most studies are based on in vitro experiments using high concn of quercetin unachievable by dietary ingestion, and because its beneficial effects on cancer are still inconclusive in animal and/or human studies.
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 580 (2005)
... Quercetin has been shown to protect low density lipoprotein (LDL) from oxidation and prevent platelet aggregation. It was also reported to inhibit the proliferation and migration of smooth muscle cells ... Quercetin was reported to significantly lower the plasma lipid, lipoprotein and hepatic cholesterol levels, inhibit the production of oxLDL produced by oxidative stress, and protect an enzyme, which can hydrolyzed specific lipid peroxides in oxidized lipoproteins and in atherosclerotic lesions ... /It/ induced endothelium-dependent vasorelaxation in rat aorta via incr nitric oxide production ... Quercetin and its glycosides were also reported to inhibit the angiotensin-converting enzyme activity, and ANG II-induced JNK activation inducing vascular smooth muscle cell (VSMC) hypertrophy ... However, some effects may not be feasible or negligible in physiological conditions, because concn of quercetin in most studies are too high to be achieved by dietary ingestion ... and beneficial effects of quercetin on cardiovascular diseases are still inconclusive in human studies ...
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 580 (2005)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
After oral administration of a single dose of 4 g quercetin to four male and two female volunteers, neither quercetin nor its conjugates was detected in the blood or urine during the first 24 hr; 53% of the dose was recovered in the feces within 72 hr. After a single intravenous injection of 100 mg quercetin to six volunteers, the blood plasma levels declined biphasically, with half-lives of 8.8 min and 2.4 hr; protein binding exceeded 98%. In the urine, 0.65% of the intravenous dose was excreted as unchanged quercetin and 7.4% as a conjugate within 9 hr; no further excretion occurred up to 24 hr ...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V73 P501 (1999)
When 14C-quercetin was administered orally to ACI rats, about 20% of the administered dose was absorbed from the digestive tract, more than 30% was decomposed to yield 14-CO2 & about 30% was excreted unchanged in feces..
PMID:6876476 UENO K ET AL; JPN J EXP MED 53(1): 41 (1983)
One male and one female volunteer were given a diet containing quercetin glucosides (64.2 mg expressed as the aglycone). The mean peak plasma concentration of quercetin was 196 ng/mL which was reached 2.9 hr after ingestion. The time-course of the plasma concentration of quercetin was biphasic, with half-lives of 3.8 hr for the distribution phase and 16.8 hr for the elimination phase. Quercetin was still present in plasma 48 hr after ingestion ... /Quercetin glucosides/
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V73 P501 (1999)
Autoradiographic analysis of a fasted rat 3 hr after administration of a single oral dose of 2.3 mg/kg (4-(14)C)quercetin showed that although most of the radiolabel remained in the digestive tract it also occurred in blood, liver, kidney, lung and ribs. After oral administration of 630 mg/kg of the labelled compound to rats, 34% of the radiolabel excreted within 24 hr ... was expired carbon dioxide, 12% in bile and 9% in urine; within 48 hr, 45% was recovered in the feces. Approximately 60% of the radiolabel in the feces was identified as unmetabolized quercetin ...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V73 P501 (1999)
For more Absorption, Distribution and Excretion (Complete) data for QUERCETIN (9 total), please visit the HSDB record page.
The glycosides are hydrolyzed in the body to corresponding aglycones, which are then further metabolized by scission of the heterocyclic ring to give 3,4-dihydroxy-phenyl-substituted acids ... The site of ring scission depends on structure ... with flavonols (quercetin) scission occurs at the 1,2 & 3,4 bonds to yield homoprotocatechuic acid ... These acids are further metabolized by beta-oxidation of acyl side-chain, o-methylation & demethylation, & aromatic dehydroxylation.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 151
o-Beta-hydroxyethylated derivatives of quercetin were isolated from urine samples & separated by HPLC. The 5,7,3',4'-tetra compd was separated from 3,7,3',4'-tetra derivative. The 7,3',4'-tri & 7'-mono compounds gave 1 common peak, separated from the peak for the 7,4'-di compd.
KUHNZ W ET AL; STUD ORG CHEM (AMSTERDAM) 11(FLAVONOIDS BIOFLAVONOIDS): 293 (1982)
After oral admin to ACI rats, the absorbed (14)C-quercetin was rapidly excreted into the bile & urine within 48 hr as the glucuronide & sulfate conjugates of (14)C-quercetin, 3'-o-monomethyl quercetin & 4'-o-monomethyl quercetin. Efficient metabolism and elimination of quercetin may be one reason for the lack of carcinogenicity in rats.
PMID:6876476 UENO K ET AL; JPN J EXP MED 53(1): 41 (1983)
The metabolites of quercetin flavonols identified in urine samples collected from two male volunteers who consumed their habitual diets for three days were 3,4-dihydroxyphenylacetic acid, meta-hydroxyphenylacetic acid, and 4-hydroxy-3-methoxyphenylacetic acid ...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V73 P501 (1999)
For more Metabolism/Metabolites (Complete) data for QUERCETIN (10 total), please visit the HSDB record page.
Quercetin has known human metabolites that include Dihydroquercetin and Mikwelianin.
Quercetin is a known human metabolite of Quercitrin and tamarixetin.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
One male and one female volunteer were given a diet containing quercetin glucosides (64.2 mg expressed as the aglycone) ... Half-lives /were/ 3.8 hr for the distribution phase and 16.8 hr for the elimination phase ... /Quercetin glucosides/
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V73 P501 (1999)
...The elimination half-life of quercetin is approx 25 hr.
Thomson Healthcare. PDR for Nutritional Supplements. Thomson Health Care Inc. Montvale, NJ. p.391 (2001)
Quercetin is a specific quinone reductase 2 (QR2) inhibitor, an enzyme (along with the human QR1 homolog) which catalyzes metabolism of toxic quinolines. Inhibition of QR2 in plasmodium may potentially cause lethal oxidative stress. The inhibition of antioxidant activity in plasmodium may contribute to killing the malaria causing parasites.
... The 5, 7, 3', 4'-hydroxyl groups on quercetin are capable of donating electrons to quench various radical oxygen species (ROS) and other radical species ... Oxygen radicals (superoxide, hydrogen peroxide, hydroxyl radicals, and other related radicals) ... are quenched by ... antioxidant systems, including antioxidant cmpd, which balance cellular redox status involved in cellular processes for cell homeostasis ... Generally, 3 criteria are considered to assess the antioxidant activity of flavonoids in vitro: first, B ring with 2 hydroxyl groups (adjacent), second, C ring with 2,3-double bond, 4-oxo, and 3-hydroxyl group, and third, A ring with 5,7-dihydroxyl groups. Quercetin meets all 3 criteria, indicating stronger antioxidant activity ... The flavonol was reported to prevent radicals from damaging carbohydrates, proteins, nucleotides, and lipids ... The glucuronide conjugates found in the plasma were also reported to have potent antioxidant activity, indicating that the activity may be retained depending on conjugation positions ...
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 579 (2005)
Cytokines such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) can induce apoptosis in colon cancer cells through engagement of death receptors. Nevertheless, evading apoptosis induced by anticancer drugs characterizes many types of cancers. This results in the need for combination therapy. In this study ... whether the flavonoid quercetin could sensitize human colon adenocarcinoma cell lines to TRAIL-induced apoptosis /was investigated/ ... Quercetin enhanced TRAIL-induced apoptosis by causing the redistribution of DR4 and DR5 into lipid rafts. Nystatin, a cholesterol-sequestering agent, prevented quercetin-induced clustering of death receptors and sensitization to TRAIL-induced apoptosis in colon adenocarcinoma cells ... Qercetin, in combination with TRAIL, triggered the mitochondrial-dependent death pathway, as shown by Bid cleavage and the release of cytochrome c to the cytosol. Together /the/ findings propose that quercetin, through its ability to redistribute death receptors at the cell surface, facilitates death-inducing signaling complex formation and activation of caspases in response to death receptor stimulation. Based on these results, this study provides a challenging approach to enhance the efficiency of TRAIL-based therapies.
PMID:17876056 Psahoulia FH et al; Mol Cancer Ther 6(9):2591-9 (2007)
Previously /the authors/ reported that isoflavone (genistein) activated bone sialoprotein (BSP) gene transcription is mediated through an inverted CCAAT box in the proximal BSP gene promoter. The present study investigates the regulation of BSP transcription in a rat osteoblast-like cell line, ROS 17/2.8 cells, by quercetin and its conjugated metabolite quercetin 3-glucuronide. Quercetin and quercetin 3-glucuronide (5 uM) increased the BSP mRNA levels at 12 hr and quercetin upregulated the Cbfa1/Runx2 mRNA expression at 12 hr. From transient transfection assays using various sized BSP promoter-luciferase constructs, quercetin increased the luciferase activity of the construct (pLUC3), including the promoter sequence nucleotides -116 to -43. Transcriptional stimulations by quercetin were almost completely abrogated in the constructs that included 2 bp mutations in the inverted CCAAT and FRE elements whereas the CCAAT-protein complex did not change after stimulation by quercetin according to gel shift assays. Quercetin increased the nuclear protein binding to the FRE and 3'-FRE. These data suggest that quercetin and quercetin 3-glucuronide increased the BSP mRNA expression, and that the inverted CCAAT and FRE elements in the promoter of the BSP gene are required for quercetin induced BSP transcription.
PMID:17243115 Kim DS et al; J Cell Biochem 101(3):790-800 (2007)
Connexin proteins form gap junctions, which permit direct exchange of cytoplasmic contents between neighboring cells. Evidence indicates that gap junctional intercellular communication (GJIC) is important for maintaining homeostasis and preventing cell transformation. Furthermore, connexins may have independent functions including tumor growth suppression. Most tumors express less connexins, have reduced GJIC and have increased growth rates compared with non-tumorigenic cells. The purpose of this study was to determine whether common flavonoids, genistein and quercetin, increase connexin43 (Cx43) levels, improve GJIC and suppress growth of a metastatic human breast tumor cell line (MDA-MB-231). Quercetin (2.5, 5 ug/mL) and genistein (0.5, 2.5, 15 ug/mL) upregulated Cx43 but failed to increase GJIC. Cx43 localized to the plasma membrane following genistein treatment (2.5, 15 ug/mL). In contrast, Cx43 aggregated in the perinuclear region following quercetin treatment (0.5, 2.5, 5, 15 ug/mL). Both genistein (15 ug/mL) and quercetin (2.5, 5, 15 ug/mL) significantly reduced MDA-MB-231 cell proliferation. In summary, genistein and quercetin increase Cx43 and suppress MDA-MB-231 cell proliferation at physiologically relevant concentrations. These results demonstrate that genistein and quercetin are potential anti-breast cancer agents.
PMID:16777995 Conklin CM et al; Carcinogenesis 28(1):93-100 (2007)
For more Mechanism of Action (Complete) data for QUERCETIN (12 total), please visit the HSDB record page.