

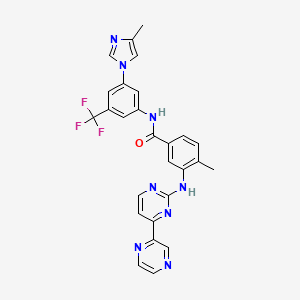

1. 4-methyl-n-(3-(4-methylimidazol-1-yl)-5-trifluoromethylphenyl)-3-(4-pyrazin-2-ylpyrimidin-2-ylamino)benzamide
2. 4-methyl-n-(3-(4-methylimidazol-1-yl)-5-trifluoromethylphenyl)-3-(4-pyrazin-2-ylpyrimidin-2-ylamino)benzamide Hydrochloride
3. Iy5511
4. Iy5511hcl
1. 926037-48-1
2. Supect
3. Iy-5511
4. Radotinib [inn]
5. Iy5511
6. Radotinib(iy-5511)
7. 4-methyl-n-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyrazin-2-ylpyrimidin-2-yl)amino]benzamide
8. I284ljy110
9. 926037-48-1 (free Base)
10. 4-methyl-n-[3-(4-methyl-1h-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-{[4-(pyrazin-2-yl)pyrimidin-2-yl]amino}benzamide
11. 4-methyl-n-(3-(4-methylimidazol-1-yl)-5-trifluoromethylphenyl)-3-(4-(pyrazin-2-yl)pyrimidin-2-ylamino)benzamide
12. Iy5511hcl
13. 4-methyl-n-(3-(4-methyl-1h-imidazol-1-yl)-5-(trifluoromethyl)phenyl)-3-((4-(pyrazin-2-yl)pyrimidin-2-yl)amino)benzamide
14. Unii-i284ljy110
15. Radotinib [mi]
16. Radotinib (iy-5511)
17. Radotinib; Iy-5511;
18. Radotinib [who-dd]
19. Gtpl7814
20. Schembl3359952
21. Chembl4297524
22. Dtxsid90239069
23. Bcp12554
24. Ex-a1035
25. Mfcd27956910
26. Nsc800989
27. S8134
28. Zinc59749972
29. Akos026750428
30. Akos032950010
31. Ccg-269914
32. Db12323
33. Iy 5511
34. Nsc-800989
35. Sb11083
36. Ncgc00390586-01
37. Ncgc00481590-01
38. Ac-30633
39. As-55884
40. Da-40474
41. Kb-146009
42. B5846
43. Ft-0700508
44. A13029
45. 2?,3?,17?-trihydroxy-7?-6-oxo-5?-androstan
46. J-690392
47. Q15269680
48. Benzamide, 4-methyl-n-(3-(4-methyl-1h-imidazol-1-yl)-5-(trifluoromethyl)phenyl)-3-((4-(2-pyrazinyl)-2-pyrimidinyl)amino)-
| Molecular Weight | 530.5 g/mol |
|---|---|
| Molecular Formula | C27H21F3N8O |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 6 |
| Exact Mass | 530.17904181 g/mol |
| Monoisotopic Mass | 530.17904181 g/mol |
| Topological Polar Surface Area | 111 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 818 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Radotinib is indicated for the treatment of different types of cancer, most notably Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) with resistance or intolerance of other Bcr-Abl tyrosine-kinase inhibitors, such as patients resistant or intolerant to imatinib.
Philadelphia chromosome positive (Ph+) leukemia is driven by the constitutive enzymatic activity of the BCR-ABL1 fusion kinase. Tyrosine kinase inhibitors (TKIs) that block the activity of BCR-ABL1 are successfully used clinically to treat chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).