

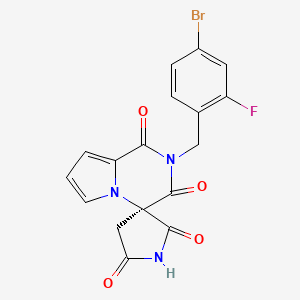

1. 2-(4-bromo-2-fluorobenzyl)-1,2,3,4-tetrahydropyrrolo(1,2-a)pyrazine-4-spiro-3'-pyrrolidine-1.2',3,5'-tetrone
2. As 3201
3. As-3201
4. Sx 3030
5. Sx 3202
6. Sx-3030
7. Sx-3202
1. 147254-64-6
2. As-3201
3. Ranirestat [inn]
4. Z26p56gftv
5. Chembl334830
6. (3r)-2'-[(4-bromo-2-fluorophenyl)methyl]spiro[pyrrolidine-3,4'-pyrrolo[1,2-a]pyrazine]-1',2,3',5-tetrone
7. Sx-3030
8. (3r)-2'-(4-bromo-2-fluorobenzyl)spiro(pyrrolidine-3,4'(1'h)-pyrrolo(1,2-a)pyrazine)-1',2,3',5(2'h)-tetrone
9. (r)-2'-(4-bromo-2-fluorobenzyl)-1'h-spiro[pyrrolidine-3,4'-pyrrolo[1,2-a]pyrazine]-1',2,3',5(2'h)-tetraone
10. Unii-z26p56gftv
11. C17h11brfn3o4
12. Raniestat
13. As 3201
14. Sx 3201
15. Ranirestat (jan/inn)
16. Ranirestat [jan]
17. Ranirestat [who-dd]
18. Schembl498993
19. Ranirestat, >=97% (hplc)
20. Dtxsid80163642
21. Zinc598422
22. Bcp14386
23. Bdbm50067407
24. Db05327
25. Sb17385
26. Sx-3201
27. Ncgc00484081-01
28. (r)-(-)-2-(4-bromo-2-fluorobenzyl)-1,2,3,4-tetrahydropyrrolo(1,2-a)pyrazine-4-spiro-3'-pyrrolidine-1,2',3,5'-tetrone
29. 2-(4-bromo-2-fluorobenzyl)-1,2,3,4-tetrahydropyrrolo(1,2-a)pyrazine-4-spiro-3'-pyrrolidine-1.2',3,5'-tetrone
30. Hy-15314
31. A13523
32. D06403
33. A901889
34. Q7293074
35. (3r)-2''-(4-bromo-2-fluorobenzyl)-1''h,2h,5h-spiro[pyrrolidine-3,4''-pyrrolo[1,2-a]pyrazine]-1'',2,3'',5(2''h)-tetrone
36. (3r)-2-(4-bromo-2-fluorobenzyl)spiro(pyrrolidine-3,4(1h)-pyrrolo(1,2-a)pyrazine)-1,2,3,5(2h)-tetrone
37. (r)-2-(4-bromo-2-fluorobenzyl)-1h-spiro[pyrrolidine-3,4-pyrrolo[1,2-a]pyrazine]-1,2,3,5(2h)-tetraone
38. Spiro(pyrrolidine-3,4'(1'h)-pyrrolo(1,2-a)pyrazine)-1',2,3',5(2'h)-tetrone, 2'-((4-bromo-2-fluorophenyl)methyl)-, (-)-
39. Spiro(pyrrolidine-3,4'(1'h)-pyrrolo(1,2-a)pyrazine)-1',2,3',5(2'h)-tetrone, 2'-((4-bromo-2-fluorophenyl)methyl)-, (3'r)-
| Molecular Weight | 420.2 g/mol |
|---|---|
| Molecular Formula | C17H11BrFN3O4 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 418.99170 g/mol |
| Monoisotopic Mass | 418.99170 g/mol |
| Topological Polar Surface Area | 88.5 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 689 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in neuropathy (diabetic).
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Ranirestat alleviates diabetic neuropathy, a complication of diabetes, by inhibiting aldose reductase and thereby inhibiting the accumulation of intracellular sorbitol that causes diabetic neuropathy. This drug has a stronger inhibitory effect and is longer acting compared to other drugs in this therapeutic area. Ranirestat showed good penetration into the nerve tissue, resulting in dose-dependent inhibition of intraneural accumulation of sorbitol and fructose in a clinical study.