
1. Ah 19065
2. Ah-19065
3. Ah19065
4. Biotidin
5. Hydrochloride, Ranitidine
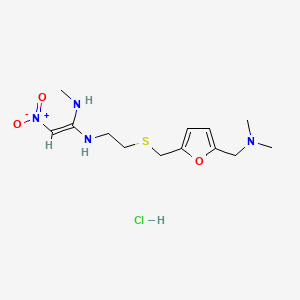
6. N (2-(((5-((dimethylamino)methyl)-2-furanyl)methyl)thio)ethyl)-n'-methyl-2-nitro-1,1-ethenediamine
7. Ranisen
8. Ranitidin
9. Ranitidine
10. Ranitidine Hydrochloride
11. Sostril
12. Zantac
13. Zantic
1. Ranitidine Hydrochloride
2. 66357-59-3
3. 71130-06-8
4. Fendibina
5. Gastridina
6. Gastrolav
7. Kuracid
8. Raniberl
9. Ranibloc
10. Alquen
11. Rani-nerton
12. Zantac
13. Nu-ranit
14. Rani-q
15. Ranitidine (hydrochloride)
16. Santanol
17. Tanidina
18. Toriol
19. Coralen
20. Quantor
21. Radin
22. Chebi:8777
23. Gastrial
24. Raniben
25. Ranibeta
26. Azantac
27. Zantac (tn)
28. (e)-1-n'-[2-[[5-[(dimethylamino)methyl]furan-2-yl]methylsulfanyl]ethyl]-1-n-methyl-2-nitroethene-1,1-diamine;hydrochloride
29. Mfcd00941509
30. {2-[({5-[(dimethylamino)methyl]furan-2-yl}methyl)sulfanyl]ethyl}[(z)-1-(methylamino)-2-nitroethenyl]amine Hydrochloride
31. Smr000653467
32. (e)-n-(2-(((5-((dimethylamino)methyl)furan-2-yl)methyl)thio)ethyl)-n-methyl-2-nitroethene-1,1-diamine
33. N-(2-((5-((dimethylamino)methyl)furfuryl)thio)ethyl)-n'-methyl-2-nitrovinylidenediamine Monohydrochloride
34. C13h22n4o3s.hcl
35. Zantic
36. Zantac;noctone
37. Sr-01000075288
38. Ah19065
39. Ah-19065
40. Prestwick_635
41. Einecs 275-207-4
42. Ranitidine (zantac)
43. Ranitidine Hydrochloride 100 Microg/ml In Acetonitrile
44. Epitope Id:127516
45. Mls001146938
46. Mls002548856
47. Chembl1092473
48. Hy-b0281a
49. Hms1568j03
50. Amy24712
51. Bcp28420
52. N-[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-n'-methyl-2-nitro-1,1-ethenediamine Hydrochloride
53. N-[2-[5-[(dimethylamino)methyl]furfurylthio]ethyl]-n'-methyl-2-nitro-1,1-ethenediamine Hydrochloride
54. Tox21_501073
55. S1801
56. Ranitidine Hydrochloride (jp17/usp)
57. Akos015895250
58. Ccg-212946
59. Lp01073
60. Nc00479
61. Ranitidine For Impurity A Identification
62. Ncgc00094351-01
63. Ncgc00261758-01
64. Ac-12743
65. N-[2-[[5-(dimethylaminomethyl)furan-2-yl]methylsulfanyl]ethyl]-n'-methyl-2-nitroethene-1,1-diamine Hydrochloride
66. Eu-0101073
67. R-101
68. R0073
69. Sw196800-3
70. Bim-0051043.0001
71. D00673
72. 357n593
73. A835435
74. Sr-01000075288-2
75. W-104534
76. (e)-n-(2-(((5-((dimethylamino)methyl)furan-2-yl)methyl)thio)ethyl)-n'-methyl-2-nitroethene-1,1-diamine Hydrochloride
77. (e)-n1'-[2-[[5-[(dimethylamino)methyl]-2-furanyl]methylthio]ethyl]-n1-methyl-2-nitroethene-1,1-diamine Hydrochloride
78. (e)-n1'-[2-[[5-[(dimethylamino)methyl]furan-2-yl]methylsulfanyl]ethyl]-n1-methyl-2-nitro-ethene-1,1-diamine Hydrochloride
79. 1,1-ethenediamine, N-(2-(((5-((dimethylamino)methyl)-2-furanyl)methyl)thio)ethyl)-n'-methyl-2-nitro-, Hydrochloride
80. N-(2-(((5-((dimethylamino)methyl)furan-2-yl)methyl)thio)ethyl)-n'-methyl-2-nitroethene-1,1-diamine Hydrochloride
81. N-(2-(((5-((dimethylamino)methyl)furan-2-yl)methyl)thio)ethyl)-n-methyl-2-nitroethene-1,1-diaminehydrochloride
82. N-(2-((5-(dimethylaminomethyl)furan-2-yl)methylsulfanyl)ethyl)-n'-methyl-2-nitroethene-1,1-diamine Hcl
83. N-[2-[[[5-[(dimethylamino)methyl]-2 -furanyl]methyl]thio]ethyl]-n'-methyl-2-nitro-1,1-e Thanediamine Hydrochloride
| Molecular Weight | 350.87 g/mol |
|---|---|
| Molecular Formula | C13H23ClN4O3S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Exact Mass | 350.1179395 g/mol |
| Monoisotopic Mass | 350.1179395 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 347 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 10 | |
|---|---|
| Drug Name | Ranitidine hydrochloride |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Tablet; Syrup; Capsule; Injectable |
| Route | Injection; Oral |
| Strength | eq 150mg base; eq 15mg base/ml; eq 75mg base; 25mg/ml; eq 300mg base; eq 25mg base/ml |
| Market Status | Over the Counter; Prescription |
| Company | Vintage Pharms; Amneal Pharms; Wockhardt; Silarx; Breckenridge Pharm; Teva; Pharm Assoc; Apotex; Torpharm; Shasun Chems; Taro; Sandoz; Par Pharm; Perrigo R And D; Svads Holdings Sa; Glenmark Generics; Amneal Pharms Ny; Ivax Sub Teva Pharms; Hi Tech Pharma |
| 2 of 10 | |
|---|---|
| Drug Name | Zantac |
| PubMed Health | Ranitidine (Injection) |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in ZANTAC 150 Tablets, ZANTAC 300 Tablets, and ZANTAC Syrup is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N-methyl-2... |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Syrup; Injectable |
| Route | Injection; Oral |
| Strength | eq 15mg base/ml; eq 25mg base/ml |
| Market Status | Prescription |
| Company | Covis Injectables; Glaxo Grp |
| 3 of 10 | |
|---|---|
| Drug Name | Zantac 150 |
| PubMed Health | Ranitidine |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in ZANTAC 150 Tablets, ZANTAC 300 Tablets, and ZANTAC Syrup is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N-methyl-2... |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 150mg base |
| Market Status | Over the Counter; Prescription |
| Company | Boehringer Ingelheim; Glaxo Grp |
| 4 of 10 | |
|---|---|
| Drug Name | Zantac 300 |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 300mg base |
| Market Status | Prescription |
| Company | Glaxo Grp |
| 5 of 10 | |
|---|---|
| Drug Name | Zantac 75 |
| Drug Label | The active ingredient in ZANTAC 150 Tablets, ZANTAC 300 Tablets, and ZANTAC Syrup is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N-methyl-2... |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 75mg base |
| Market Status | Over the Counter |
| Company | Boehringer Ingelheim |
| 6 of 10 | |
|---|---|
| Drug Name | Ranitidine hydrochloride |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Tablet; Syrup; Capsule; Injectable |
| Route | Injection; Oral |
| Strength | eq 150mg base; eq 15mg base/ml; eq 75mg base; 25mg/ml; eq 300mg base; eq 25mg base/ml |
| Market Status | Over the Counter; Prescription |
| Company | Vintage Pharms; Amneal Pharms; Wockhardt; Silarx; Breckenridge Pharm; Teva; Pharm Assoc; Apotex; Torpharm; Shasun Chems; Taro; Sandoz; Par Pharm; Perrigo R And D; Svads Holdings Sa; Glenmark Generics; Amneal Pharms Ny; Ivax Sub Teva Pharms; Hi Tech Pharma |
| 7 of 10 | |
|---|---|
| Drug Name | Zantac |
| PubMed Health | Ranitidine (Injection) |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in ZANTAC 150 Tablets, ZANTAC 300 Tablets, and ZANTAC Syrup is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N-methyl-2... |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Syrup; Injectable |
| Route | Injection; Oral |
| Strength | eq 15mg base/ml; eq 25mg base/ml |
| Market Status | Prescription |
| Company | Covis Injectables; Glaxo Grp |
| 8 of 10 | |
|---|---|
| Drug Name | Zantac 150 |
| PubMed Health | Ranitidine |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | The active ingredient in ZANTAC 150 Tablets, ZANTAC 300 Tablets, and ZANTAC Syrup is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N-methyl-2... |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 150mg base |
| Market Status | Over the Counter; Prescription |
| Company | Boehringer Ingelheim; Glaxo Grp |
| 9 of 10 | |
|---|---|
| Drug Name | Zantac 300 |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 300mg base |
| Market Status | Prescription |
| Company | Glaxo Grp |
| 10 of 10 | |
|---|---|
| Drug Name | Zantac 75 |
| Drug Label | The active ingredient in ZANTAC 150 Tablets, ZANTAC 300 Tablets, and ZANTAC Syrup is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N-methyl-2... |
| Active Ingredient | Ranitidine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 75mg base |
| Market Status | Over the Counter |
| Company | Boehringer Ingelheim |
Histamine H2 Antagonists
Drugs that selectively bind to but do not activate histamine H2 receptors, thereby blocking the actions of histamine. Their clinically most important action is the inhibition of acid secretion in the treatment of gastrointestinal ulcers. Smooth muscle may also be affected. Some drugs in this class have strong effects in the central nervous system, but these actions are not well understood. (See all compounds classified as Histamine H2 Antagonists.)
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)