

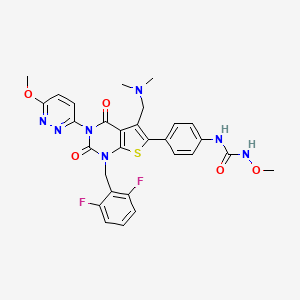

1. 1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno(2,3-d)pyrimidin-6-yl)phenyl)-3-methoxyurea
2. Orgovyx
3. Tak 385
4. Tak-385
5. Tak385
1. 737789-87-6
2. Tak-385
3. Orgovyx
4. Tak 385
5. 1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-3-methoxyurea
6. Chembl1800159
7. P76b05o5v6
8. 1-[4-[1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxothieno[2,3-d]pyrimidin-6-yl]phenyl]-3-methoxyurea
9. Urea, N-(4-(1-((2,6-difluorophenyl)methyl)-5-((dimethylamino)methyl)-1,2,3,4-tetrahydro-3-(6-methoxy-3-pyridazinyl)-2,4-dioxothieno(2,3-d)pyrimidin-6-yl)phenyl)-n'-methoxy-
10. Relugolix [usan:inn]
11. Unii-p76b05o5v6
12. Tak385
13. Tak-385/tak385
14. Relugolix (jan/inn)
15. Relugolix [inn]
16. Relugolix [jan]
17. Relugolix(tak-385)
18. Relugolix [usan]
19. Relugolix [who-dd]
20. Schembl778416
21. Gtpl5586
22. Relugolix [orange Book]
23. C29h27f2n7o5s
24. Dtxsid40224167
25. Rvt-601
26. Amy27916
27. Bcp21587
28. Ex-a1083
29. Myfembree Component Relugolix
30. Tak-385; Rvt-601
31. Bdbm50347982
32. Mfcd25976856
33. S5808
34. Zinc43206033
35. Cs-5917
36. Db11853
37. Relugolix Component Of Myfembree
38. Sb16721
39. Ac-35863
40. Bs-18032
41. Hy-16474
42. N-(4-(1-((2,6-difluorophenyl)methyl)-5-((dimethylamino)methyl)-1,2,3,4-tetrahydro-3-(6-methoxy-3-pyridazinyl)-2,4-dioxothieno(2,3-d)pyrimidin-6-yl)phenyl)-n'-methoxyurea
43. D10888
44. A857822
45. Ryeqo (relugolix-estradiol-norethisterone Acetate)
46. Q21099000
47. 1-(4-(1-((2,6-difluorophenyl)methyl)-5-((dimethylamino)methyl)-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno(2,3-d)pyrimidin-6-yl)phenyl)-3-methoxyurea
48. 1-[4-[1-[(2,6-difluorophenyl)methyl]-5-(dimethylaminomethyl)-3-(6-methoxypyridazin-3-yl)-2,4-dioxothieno[4,5-e]pyrimidin-6-yl]phenyl]-3-methoxyurea
49. 1-[4-[1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxothieno[2,3-d]pyrimidin-6-yl]phenyl]-3-methoxyurea;relugolix
50. 1-{4-[1-(2,6-difluorobenzyl)-5-dimethylaminomethyl-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea
| Molecular Weight | 623.6 g/mol |
|---|---|
| Molecular Formula | C29H27F2N7O5S |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 9 |
| Exact Mass | 623.17624448 g/mol |
| Monoisotopic Mass | 623.17624448 g/mol |
| Topological Polar Surface Area | 158 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1010 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Relugolix is indicated for the treatment of adult patients with advanced prostate cancer. In a combination product with [estradiol] and [norethindrone], relugolix is indicated for the once-daily treatment for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women.
Orgovyx is indicated for the treatment of adult patients with advanced hormone-sensitive prostate cancer .
Approximately 56% of patients achieved castrate-level testosterone concentrations (<50 ng/dL) by day 4 of therapy and 97% of patients maintain these levels through 48 weeks of therapy. Relugolix requires once-daily oral administration to maintain the desired testosterone concentrations. Androgen deprivation therapies may prolong the QTc interval and should therefore be used with caution in patients having a high baseline risk of QTc prolongation, such as those with electrolyte abnormalities, congestive heart failure, or using other medications known to prolong the QTc interval. Based on its mechanism of action and data from animal studies, relugolix may result in fetal harm if administered to pregnant females - male patients with female partners should be advised to use effective contraception throughout therapy and for 2 weeks following cessation of therapy to prevent inadvertent fetal exposure.
Androgen Antagonists
Compounds which inhibit or antagonize the biosynthesis or actions of androgens. (See all compounds classified as Androgen Antagonists.)
L02BX
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BX - Other hormone antagonists and related agents
L02BX04 - Relugolix
Absorption
The Cmax and AUC of orally-administered relugolix increase proportionally following single doses - in contrast, with repeat dosing the AUC remains proportional to the dose while the Cmax increases greater than proportionally to the dose. Following the administration of 120mg once daily, the steady-state AUC and Cmax of relugolix were 407 ( 168) ng.hr/mL and 70 ( 65) ng/mL, respectively. The absolute oral bioavailability of relugolix is approximately 12% and the median Tmax following oral administration is 2.25 hours.
Route of Elimination
Approximately 81% of an orally administered dose was recovered in the feces, of which 4.2% was unchanged parent drug, while 4.1% of the dose was recovered in the urine, of which 2.2% remained unchanged.
Clearance
The average renal clearance of relugolix is 8 L/h with a total clearance of 26.4 L/h.
Relugolix is metabolized mainly by the CYP3A subfamily of P450 enzymes, with a smaller contribution by CYP2C8.
The average effective half-life of relugolix is 25 hours, while the average terminal elimination half-life is 60.8 hours.
The pathogenesis and progression of prostate cancer appear driven, at least in part, by the effects of testosterone. Androgen deprivation has been demonstrated to result in cell death and tumor regression in many well-differentiated prostate cancer cell lines - for this reason, androgen deprivation therapy (ADT) has become a standard in the treatment of prostate cancer, particularly in advanced disease. Testosterone production in males is carried out in the Leydig cells of testes and is stimulated by luteinizing hormone (LH), which itself is produced in the pituitary gland following the binding of gonadotropin-releasing hormone (GnRH) to corresponding GnRH receptors. Relugolix is a competitive antagonist of these GnRH receptors, thereby decreasing the release of LH and, ultimately, testosterone.
