


1. Heliotridine
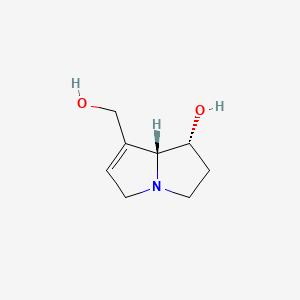
2. Retronecine, (1s-cis)-isomer
3. Retronecine, Monohydrochloride, (1s-cis)-isomer
4. Retronecine, Monohydrochloride, (1s-trans)-isomer
1. 480-85-3
2. Senecifolinene
3. Retronecin
4. (+)-retronecine
5. Ccris 5776
6. (1r,7ar)-7-(hydroxymethyl)-2,3,5,7a-tetrahydro-1h-pyrrolizin-1-ol
7. (1r,8r)-7-(hydroxymethyl)-2,3,5,8-tetrahydro-1h-pyrrolizin-1-ol
8. 2p5723m6ii
9. (1r,7ar)-2,3,5,7a-tetrahydro-1-hydroxy-1h-pyrrolizine-7-methanol
10. ( )-retronecine
11. Hsdb 3567
12. Unii-2p5723m6ii
13. Retronecine [mi]
14. 1h-pyrrolizine-7-methanol, 2,3,5,7a-beta-tetrahydro-1-alpha-hydroxy-
15. Retronecine [hsdb]
16. Schembl673883
17. Chebi:8821
18. Retronecine, Analytical Standard
19. Dtxsid401020156
20. Hy-n8419
21. Zinc1996064
22. Mfcd01684783
23. Stl564628
24. 1h-pyrrolizine-7-methanol, 2,3,5,7a-tetrahydro-1-hydroxy-, (1r-trans)-
25. Akos003672869
26. Cs-0144131
27. C06177
28. Q3933789
| Molecular Weight | 155.19 g/mol |
|---|---|
| Molecular Formula | C8H13NO2 |
| XLogP3 | -1.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 155.094628657 g/mol |
| Monoisotopic Mass | 155.094628657 g/mol |
| Topological Polar Surface Area | 43.7 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 191 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Lactating rats dosed with (3)H-retronecine excreted within 3 hr approx 0.08% of the applied radioactivity in the milk mainly as yet not identified water soluble retronecine-derived metabolites and approx 0.02% as unchanged pyrrolizidine alkaloids (pas). Highest tissue levels of pas and metabolites, 6 hr after admin were found in liver and lungs.
PMID:6623523 Luthy J et al; Toxicol Lett 17(3-4): 283 (1983)
Steric hindrance around the ester groups was the major factor inhibiting hydrolysis of pyrrolizidine alkaloids. Enzymic hydrolysis of retronecine di-isovalerate occurred primarily at the allylic 9-ester group. The results supported the view that a factor contributing to the lower hepatotoxicity of semisynthetic retronecine diesters compared with some natural pyrrolizidine alkaloids, is their greater susceptibility to detoxication by hydrolysis.
Mattocks A; Toxicol Lett 14(1-2) 111 (1982)
Levels of pyrrolic metabolites were measured in the livers of rats given some pyrrolizidine alkaloids and semisynthetic derivatives. Structural and chemical features favoring the formation of such metabolites were defined. The most important of these were steric hindrance or chemical properties giving resistance to ester hydrolysis; lipophilic character, allowing access to hepatic microsomal enzymes; a conformation favoring microsomal oxidation of the pyrroline ring in preference to n-oxidation.
PMID:7226276 Mattocks A; Chem-Biol Interact 35(3): 301 (1981)
Levels of pyrrolic metabolites were measured in the livers of rats given some pyrrolizidine alkaloids and semisynthetic derivatives. ... Heliotridine-based alkaloids gave more pyrrole than similar retronecine esters, heliotridine ditiglate gave less pyrrole than retronecine ditiglate because the former was more open to hydrolytic attack.
PMID:7226276 Mattocks AR; Chem-Biol Interact 35(3): 301-310 (1981)