


1. Wf 1360b
2. Wf 1360c
3. Wf 1360e
4. Wf 1360f
5. Wf-1360
6. Wf1360
1. Antibiotic Wf-1360
2. Wf-1360
3. 90996-54-6
4. Chebi:72590
5. C1v1y784e4
6. Nsc-332598
7. Fr-900216
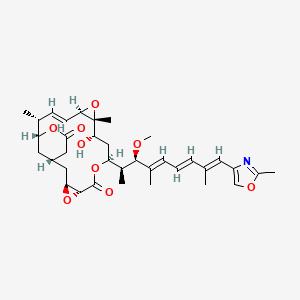
8. (1s,3s,5r,8s,10s,11r,13r,14e,16r,17r)-10-hydroxy-8-[(2s,3r,4e,6e,8e)-3-methoxy-4,8-dimethyl-9-(2-methyl-1,3-oxazol-4-yl)nona-4,6,8-trien-2-yl]-11,16-dimethyl-4,7,12,18-tetraoxatetracyclo[15.3.1.0(3,5).0(11,13)]henicos-14-ene-6,19-dione
9. (1s,3s,5r,8s,10s,11r,13r,16r,17r,e)-10-hydroxy-8-((2s,3r,4e,6e,8e)-3-methoxy-4,8-dimethyl-9-(2-methyloxazol-4-yl)nona-4,6,8-trien-2-yl)-11,16-dimethyl-4,7,12,18-tetraoxatetracyclo[15.3.1.03,5.011,13]henicos-14-ene-6,19-dione
10. Nsc 332598
11. Brn 5692896
12. Unii-c1v1y784e4
13. Nsc332598
14. Rhizoxin [mi]
15. Schembl15955
16. Chembl379989
17. Dtxsid20897515
18. Crc-87/01
19. E-87/010
20. Q10861060
21. (1s,3s,5r,8s,10s,11r,13r,14e,16r,17r)-10-hydroxy-8-[(2s,3r,4e,6e,8e)-3-methoxy-4,8-dimethyl-9-(2-methyl-1,3-oxazol-4-yl)nona-4,6,8-trien-2-yl]-11,16-dimethyl-4,7,12,18-tetraoxatetracyclo[15.3.1.03,5.011,13]henicos-14-ene-6,19-dione
22. (1s-(1r*,3r*,5s*,8r*(1r*,2s*,3e,5e,7e),10r*,11s*,13s*,14e,16s*,17s*))-10-hydroxy-8-(2-methoxy-1,3,7-trimethyl-8-(2-methyl-4-oxazolyl)-3,5,7-octatrienyl)-11,16-dimethyl-4,7,12,18-tetraoxatetracyclo(15.3.1.03,5.011,13)heneicos-14-ene-6,19-dione
23. 4,7,12,18-tetraoxatetracyclo(15.3.1.03,5.011,13)heneicos-14-ene-6,19-dione, 10-hydroxy-8-(2-methoxy-1,3,7-trimethyl-8-(2-methyl-4-oxazolyl)-3,5,7-octatrienyl)-11,16-dimethyl-, (1s-(1r*,3r*,5s*,8r*(1r*,2s*,3e,5e,7e),10r*,11s*,13s*,14e,16s*,17s*))-
24. 4,7,12,18-tetraoxatetracyclo[15.3.1.03,5.011,13]heneicos-14-ene-6,19-dione, 10-hydroxy-8-[(1s,2r,3e,5e,7e)-2-methoxy-1,3,7-trimethyl-8-(2-methyl-4-oxazolyl)-3,5,7-octatrienyl]-11,16-dimethyl-, (1s,3s,5r,8s,10s,11r,13r,16s,17r)-
25. Hydroxy-[(1s,2r,3e,5e,7e)-2-methoxy-1,3,7-trimethyl-8-(2-methyloxazol-4-yl)octa-3,5,7-trienyl]-dimethyl-[?]dione
| Molecular Weight | 625.7 g/mol |
|---|---|
| Molecular Formula | C35H47NO9 |
| XLogP3 | 5.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 625.32508208 g/mol |
| Monoisotopic Mass | 625.32508208 g/mol |
| Topological Polar Surface Area | 133 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 1210 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 11 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)