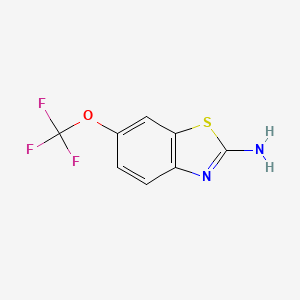

1. 2 Amino 6 Trifluoromethoxybenzothiazole
2. 2-amino-6-trifluoromethoxybenzothiazole
3. Pk 26124
4. Pk-26124
5. Pk26124
6. Rilutek
7. Rp 54274
8. Rp-54274
9. Rp54274
1. 1744-22-5
2. Rilutek
3. 2-amino-6-(trifluoromethoxy)benzothiazole
4. 6-(trifluoromethoxy)benzo[d]thiazol-2-amine
5. 6-(trifluoromethoxy)-1,3-benzothiazol-2-amine
6. Rp-54274
7. 2-amino-6-trifluoromethoxybenzothiazole
8. 2-amino-6-(trifluoromethoxy)benzo[d]thiazole
9. 2-benzothiazolamine, 6-(trifluoromethoxy)-
10. Pk-26124
11. Rp 54274
12. Tiglutik
13. 2-amino-6-(trifluoromethoxy)-benzothiazole
14. Riluzole (rilutek)
15. Amino-2 Trifluoromethoxy-6 Benzothiazole
16. Mls000069369
17. 6-trifluoromethoxy-benzothiazol-2-ylamine
18. 2-benzothiazolamine,6-(trifluoromethoxy)-
19. Chembl744
20. Nsc-753433
21. Nsc-759823
22. Smr000058231
23. 7lj087rs6f
24. Chebi:8863
25. Riluzol
26. 2-amino-6-(trifluoromethoxy)-1,3-benzothiazole
27. Benzothiazole, 2-amino-6-trifluoromethoxy-
28. Bhv-0223
29. Ncgc00015882-09
30. Riluzolum
31. Riluzol [inn-spanish]
32. Riluzolum [inn-latin]
33. Dsstox_cid_25192
34. Dsstox_rid_80739
35. Dsstox_gsid_45192
36. C8h5f3n2os
37. Rilutek (tn)
38. Cas-1744-22-5
39. Amino-2 Trifluoromethoxy-6 Benzothiazole [french]
40. Unii-7lj087rs6f
41. 2-amino-6-(trifluoromethoxyl)benzothiazole
42. Riluzole, Solid
43. Riluzole [usan:usp:inn:ban]
44. Riluzole- Bio-x
45. Bf-37
46. Albb-006046
47. Mfcd00210213
48. Prestwick-03a08
49. Exservan
50. Pk26124
51. 6-(trifluoromethoxy)-2-benzothiazolamine
52. Tocris-0768
53. Pk 26124
54. 6-trifluoromethoxybenzothiazole-2-yl-amine
55. Opera_id_548
56. Riluzole [usan]
57. Lopac-r-116
58. Riluzole [inn]
59. Riluzole [jan]
60. Riluzole [mi]
61. Riluzole-13c-15n2
62. Prestwick0_000167
63. Prestwick1_000167
64. Prestwick2_000167
65. Prestwick3_000167
66. Spectrum2_000550
67. Riluzole [mart.]
68. Biomol-nt_000245
69. Riluzole [usp-rs]
70. Riluzole [who-dd]
71. Cid_5070
72. Riluzole (jan/usp/inn)
73. Riluzole [ema Epar]
74. Lopac0_001064
75. Schembl78905
76. Bspbio_000033
77. Bidd:gt0055
78. Spbio_000599
79. Spbio_001954
80. Riluzole [orange Book]
81. Bpbio1_000037
82. Bpbio1_000837
83. Gtpl2326
84. Zinc6481
85. Riluzole [usp Impurity]
86. Dtxsid3045192
87. Riluzole [usp Monograph]
88. Bdbm30705
89. Bio1_000416
90. Bio1_000905
91. Bio1_001394
92. Hms1773g08
93. Hms2089o19
94. Hms2094g07
95. Hms2233e14
96. Hms3263e10
97. Hms3371a09
98. Hms3657e13
99. Pharmakon1600-01505348
100. Amy14166
101. Bcp02142
102. Hy-b0211
103. Riluzole - Cas 1744-22-5
104. Tiglutik (thickened Oral Suspension)
105. Tox21_110252
106. Tox21_501064
107. Ac-730
108. Bbl013272
109. Ccg-39528
110. Nsc753433
111. Nsc759823
112. S1614
113. Stk503686
114. Akos000265071
115. Tox21_110252_1
116. Db00740
117. Ks-5231
118. Lp01064
119. Nsc 753433
120. Nsc 759823
121. Sdccgsbi-0051034.p003
122. 2-amino-6-trifluoromethoxy-benzothiazole
123. 6-trifluoromethoxy-2-amino-benzothiazole
124. Ncgc00015882-01
125. Ncgc00015882-02
126. Ncgc00015882-03
127. Ncgc00015882-04
128. Ncgc00015882-05
129. Ncgc00015882-06
130. Ncgc00015882-07
131. Ncgc00015882-08
132. Ncgc00015882-10
133. Ncgc00015882-11
134. Ncgc00015882-12
135. Ncgc00015882-13
136. Ncgc00015882-15
137. Ncgc00015882-28
138. Ncgc00023141-02
139. Ncgc00023141-04
140. Ncgc00023141-05
141. Ncgc00023141-06
142. Ncgc00261749-01
143. 6-(trifluoromethoxy)-2-aminobenzothiazole
144. 6-trifluoromethoxybenzo[d]thiazol-2-amine
145. Br164340
146. Sbi-0051034.p002
147. 2-amino-6-(trifluoromethoxy) Benzothiazole
148. Db-030335
149. Eu-0101064
150. Ft-0611194
151. R1174
152. Sw196805-4
153. En300-23782
154. 6-trifluoromethoxy-1,3-benzothiazol-2-ylamine
155. C07937
156. D00775
157. Vu0239571-11
158. 744r225
159. Q415744
160. Sr-01000002997-3
161. Brd-k21283037-001-02-5
162. Brd-k21283037-003-03-9
163. Brd-k21283037-003-06-2
164. F3282-0020
165. Z166605314
166. Riluzole, United States Pharmacopeia (usp) Reference Standard
167. 2-amino-6-(trifluoromethoxy)-1,3-benzothiazole;2-amino-6-(trifluoromethoxy)benzothiazole
| Molecular Weight | 234.20 g/mol |
|---|---|
| Molecular Formula | C8H5F3N2OS |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | 234.00746845 g/mol |
| Monoisotopic Mass | 234.00746845 g/mol |
| Topological Polar Surface Area | 76.4 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 238 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Rilutek |
| PubMed Health | Riluzole (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | RILUTEK (riluzole) is a member of the benzothiazole class. Chemically, riluzole is 2-amino-6-(trifluoromethoxy)benzothiazole. Its molecular formula is C8H5F3N2OS and its molecular weight is 234.2. Its structural formula is as follows:Riluzole is a... |
| Active Ingredient | Riluzole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Covis Pharma Sarl |
| 2 of 4 | |
|---|---|
| Drug Name | Riluzole |
| PubMed Health | Riluzole (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | RILUTEK (riluzole) is a member of the benzothiazole class. Chemically, riluzole is 2-amino-6-(trifluoromethoxy)benzothiazole. Its molecular formula is C8H5F3N2OS and its molecular weight is 234.2. Its structural formula is as follows:Riluzole is a... |
| Active Ingredient | Riluzole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Apotex; Sun Pharm Inds; Glenmark Generics; Impax Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Rilutek |
| PubMed Health | Riluzole (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | RILUTEK (riluzole) is a member of the benzothiazole class. Chemically, riluzole is 2-amino-6-(trifluoromethoxy)benzothiazole. Its molecular formula is C8H5F3N2OS and its molecular weight is 234.2. Its structural formula is as follows:Riluzole is a... |
| Active Ingredient | Riluzole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Covis Pharma Sarl |
| 4 of 4 | |
|---|---|
| Drug Name | Riluzole |
| PubMed Health | Riluzole (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | RILUTEK (riluzole) is a member of the benzothiazole class. Chemically, riluzole is 2-amino-6-(trifluoromethoxy)benzothiazole. Its molecular formula is C8H5F3N2OS and its molecular weight is 234.2. Its structural formula is as follows:Riluzole is a... |
| Active Ingredient | Riluzole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Apotex; Sun Pharm Inds; Glenmark Generics; Impax Labs |
For the treatment of amyotrophic lateral sclerosis (ALS, Lou Gehrig's Disease)
FDA Label
Investigated for use/treatment in atopic dermatitis.
Rilutek is indicated to extend life or the time to mechanical ventilation for patients with amyotrophic lateral sclerosis (ALS).
Clinical trials have demonstrated that Rilutek extends survival for patients with ALS.
Survival was defined as patients who were alive, not intubated for mechanical ventilation and tracheotomy-free.
There is no evidence that Rilutek exerts a therapeutic effect on motor function, lung function, fasciculations, muscle strength and motor symptoms.
Rilutek has not been shown to be effective in the late stages of ALS.
Safety and efficacy of Rilutek has only been studied in ALS. Therefore, Rilutek should not be used in patients with any other form of motor-neurone disease.
Riluzole, a member of the benzothiazole class, is indicated for the treatment of patients with amyotrophic lateral sclerosis (ALS). Riluzole extends survival and/or time to tracheostomy. It is also neuroprotective in various in vivo experimental models of neuronal injury involving excitotoxic mechanisms. The etiology and pathogenesis of amyotrophic lateral sclerosis (ALS) are not known, although a number of hypotheses have been advanced. One hypothesis is that motor neurons, made vulnerable through either genetic predisposition or environmental factors, are injured by glutamate. In some cases of familial ALS the enzyme superoxide dismutase has been found to be defective.
BF-37 interferes directly with cellular processes of the immune system of the skin, thereby diminishing the inflammation that underlies the reddening and itching.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Excitatory Amino Acid Antagonists
Drugs that bind to but do not activate excitatory amino acid receptors, thereby blocking the actions of agonists. (See all compounds classified as Excitatory Amino Acid Antagonists.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
N07XX02
N07XX02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N07 - Other nervous system drugs
N07X - Other nervous system drugs
N07XX - Other nervous system drugs
N07XX02 - Riluzole
Absorption
Riluzole is well-absorbed (approximately 90%), with average absolute oral bioavailability of about 60% (CV=30%). A high fat meal decreases absorption, reducing AUC by about 20% and peak blood levels by about 45%.
Riluzole is extensively metabolized to six major and a number of minor metabolites, which have not all been identified to date. Metabolism is mostly hepatic, consisting of cytochrome P450–dependent hydroxylation and glucuronidation. CYP1A2 is the primary isozyme involved in N-hydroxylation; CYP2D6, CYP2C19, CYP3A4, and CYP2E1 are considered unlikely to contribute significantly to riluzole metabolism in humans.
Riluzole has known human metabolites that include 4-hydroxy-riluzole, 5-hydroxy-riluzole, 7-hydroxy-riluzole, and N-Hydroxyriluzole.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The mean elimination half-life of riluzole is 12 hours (CV=35%) after repeated doses.
The mode of action of riluzole is unknown. Its pharmacological properties include the following, some of which may be related to its effect: 1) an inhibitory effect on glutamate release (activation of glutamate reuptake), 2) inactivation of voltage-dependent sodium channels, and 3) ability to interfere with intracellular events that follow transmitter binding at excitatory amino acid receptors.
BF-37 inhibits the proliferation of T-cells. An increased proliferation of these immune cells is believed to cause atopic dermatitis. Biofrontera assumes that BF-37 interferes directly with cellular processes of the immune systems of the skin, thereby diminishing the inflammation that underlines the reddening and itching.