

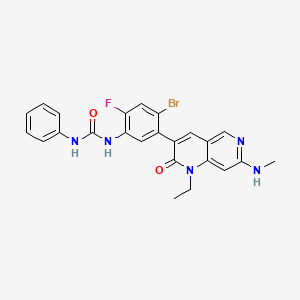

1. 1-(4-bromo-5-(1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl)-2-fluorophenyl)-3-phenylurea
2. Urea, N-(4-bromo-5-(1-ethyl-1,2-dihydro-7-(methylamino)-2-oxo-1,6-naphthyridin-3-yl)-2-fluorophenyl)-n'-phenyl-
1. 1442472-39-0
2. Dcc-2618
3. N-{4-bromo-5-[1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3- Yl]-2-fluorophenyl}-n'-phenylurea
4. Urea, N-[4-bromo-5-[1-ethyl-1,2-dihydro-7-(methylamino)-2-oxo-1,6- Naphthyridin-3-yl]-2-fluorophenyl]-n'-phenyl-39-0
5. Ripretinib Free Base
6. Ripretinib [usan]
7. Ripretinib (dcc-2618)
8. 9xw757o13d
9. Ripretinib (usan)
10. 1442472-39-0 (free Base)
11. 1-(4-bromo-5-(1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl)-2-fluorophenyl)-3-phenylurea
12. Qinlock
13. 1-[4-bromo-5-[1-ethyl-7-(methylamino)-2-oxo-1,6-naphthyridin-3-yl]-2-fluorophenyl]-3-phenylurea
14. N-(4-bromo-5-(1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl)-2-fluorophenyl)-n'-phenylurea
15. Urea, N-(4-bromo-5-(1-ethyl-1,2-dihydro-7-(methylamino)-2-oxo-1,6-naphthyridin-3-yl)-2-fluorophenyl)-n'-phenyl-
16. Quinlock
17. Qinlock (tn)
18. Ripretinib [mi]
19. Ripretinib [inn]
20. Ripretinib [who-dd]
21. Unii-9xw757o13d
22. Gtpl9175
23. Chembl4216467
24. Ripretinib [orange Book]
25. Schembl14999718
26. Dcc2618
27. Dtxsid201027956
28. Bcp29218
29. Ex-a4883
30. S8757
31. At18473
32. Db14840
33. Ac-36722
34. Hy-112306
35. Cs-0044835
36. D11353
37. Dcc 2618;dcc2618;kit/pdgfr Inhibitor;ripretinib
| Molecular Weight | 510.4 g/mol |
|---|---|
| Molecular Formula | C24H21BrFN5O2 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 509.08627 g/mol |
| Monoisotopic Mass | 509.08627 g/mol |
| Topological Polar Surface Area | 86.4 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 746 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ripretinib is indicated to treat adults diagnosed with advanced gastrointestinal stromal tumor (GIST) who have had prior therapy with at least 3 kinase inhibitors, including with [imatinib].
FDA Label
Qinlock is indicated for the treatment of adult patients with advanced gastrointestinal stromal tumour (GIST) who have received prior treatment with three or more kinase inhibitors, including imatinib.
As a broad-spectrum kinase inhibitor, ripretinib inhibits various gene mutations, increasing progression-free survival in patients with advanced gastrointestinal stromal tumors (GIST). It is effective in treating mutations that are resistant to chemotherapy with other kinase inhibitors, such as imatinib. Ripretinib has the propensity to cause cardiac dysfunction and new primary cutaneous malignancy. It is important to measure cardiac ejection fraction before and during treatment as well as to perform regular dermatological assessments.
L01
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EX - Other protein kinase inhibitors
L01EX19 - Ripretinib
Absorption
Ripretinib is absorbed in the gastrointestinal tract and Tmax is achieved in 4 hours, with steady-state concentrations reached within 14 days.
Route of Elimination
Ripretinib is 34% excreted in the feces and 0.2% excreted in the urine.
Volume of Distribution
The mean volume of distribution of ripretinib is 307 L.
Clearance
The mean apparent clearance of ripretinib is 15.3 L/hour.
Ripretinib is metabolized by the CYP3A subfamily of enzymes with contributions from CYP2D6 and CYP2E1 to its active metabolite, DP-5439.
The average half-life of ripretinib is 14.8 hours.
Protein kinases play important roles in cellular function, and their dysregulation can lead to carcinogenesis. Ripretinib inhibits protein kinases including wild type and mutant platelet-derived growth factor receptor A (PDGFRA) and KIT that cause the majority of gastrointestinal stromal tumor (GIST). In vitro, ripretinib has been shown to inhibit PDGFRB, BRAF, VEGF, and TIE2 genes. Ripretinib binds to KIT and PDGFRA receptors with mutations on the exons 9, 11, 13, 14, 17 and 18 (for KIT mutations), and exons 12, 14 and 18 (for PDGFRA mutations). The switch pocket of a protein kinase is normally bound to the activation loop, acting as an on-off switch of a kinase. Ripretinib boasts a unique dual mechanism of action of binding to the kinase switch pocket as well as the activation loop, thereby turning off the kinase and its ability to cause dysregulated cell growth.