


1. Du 21220
2. Du-21220
3. Du21220
4. Hydrochloride, Ritodrine
5. Pre Par
6. Pre-par
7. Prepar
8. Ritodrine Hydrochloride
9. Yutopar
1. Ritodrina [inn-spanish]
2. Ritodrinium [inn-latin]
3. 26652-09-5
4. Du-21220
5. Prepar
6. P-hydroxy-alpha-(1-((p-hydroxyphenethyl)amino)ethyl)benzyl Alcohol
7. Mls002153782
8. Ritodrine (usan/inn)
9. Smr001233166
10. (?)-ritodrine
11. Prestwick0_000349
12. Prestwick1_000349
13. Prestwick2_000349
14. Prestwick3_000349
15. Chembl785
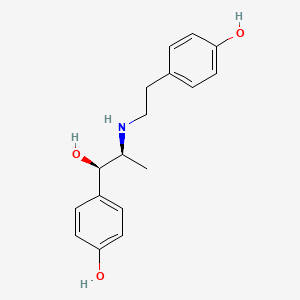
16. 4-[(1r,2s)-1-hydroxy-2-{[2-(4-hydroxyphenyl)ethyl]amino}propyl]phenol
17. Schembl34194
18. Bspbio_000417
19. Cid_31728
20. Spbio_002338
21. Bpbio1_000459
22. Chebi:8872
23. Gtpl7294
24. Dtxsid7048534
25. Bdbm97162
26. Zinc57480
27. Db00867
28. Ncgc00179566-01
29. Ncgc00179566-03
30. C07239
31. D02359
32. Q5577596
33. Brd-k51465424-003-03-6
34. 4-(2-{[(1r,2s)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-yl]amino}ethyl)phenol
| Molecular Weight | 287.35 g/mol |
|---|---|
| Molecular Formula | C17H21NO3 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 287.15214353 g/mol |
| Monoisotopic Mass | 287.15214353 g/mol |
| Topological Polar Surface Area | 72.7 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 284 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment and prophylaxis of premature labour
Beta-2 adrenergic receptors are located at sympathetic neuroeffector junctions of many organs, including uterus. Ritodrine is beta-2 adrenergic agonist. It stimulates beta-2 adrenergic receptor, increases cAMP level and decreases intracellular calcium concentration. The decrease of calcium concentration leads to a relaxation of uterine smooth muscle and, therefore, a decrease in premature uterine contractions.
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Adrenergic beta-2 Receptor Agonists
Compounds bind to and activate ADRENERGIC BETA-2 RECEPTORS. (See all compounds classified as Adrenergic beta-2 Receptor Agonists.)
Tocolytic Agents
Drugs that prevent preterm labor and immature birth by suppressing uterine contractions (TOCOLYSIS). Agents used to delay premature uterine activity include magnesium sulfate, beta-mimetics, oxytocin antagonists, calcium channel inhibitors, and adrenergic beta-receptor agonists. The use of intravenous alcohol as a tocolytic is now obsolete. (See all compounds classified as Tocolytic Agents.)
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02C - Other gynecologicals
G02CA - Sympathomimetics, labour repressants
G02CA01 - Ritodrine
Hepatic, by both the mother and fetus
1.7-2.6 hours
Ritodrine is beta-2 adrenergic agonist. It binds to beta-2 adrenergic receptors on outer membrane of myometrial cell, activates adenyl cyclase to increase the level of cAMP which decreases intracellular calcium and leads to a decrease of uterine contractions.