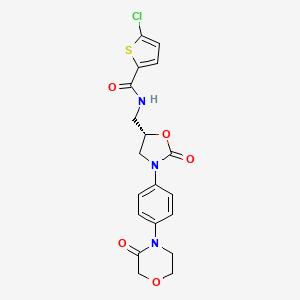

1. 5-chloro-n-(((5s)-2-oxo-3-(4-(3-oxomorpholin-4-yl)phenyl)-1,3-oxazolidin-5-yl)methyl)thiophene-2-carboxamide
2. Bay 59 7939
3. Bay 59-7939
4. Bay 597939
5. Xarelto
1. 366789-02-8
2. Xarelto
3. Bay 59-7939
4. (s)-5-chloro-n-((2-oxo-3-(4-(3-oxomorpholino)phenyl)oxazolidin-5-yl)methyl)thiophene-2-carboxamide
5. Bay59-7939
6. Bay-59-7939
7. 9ndf7jz4m3
8. C19h18cln3o5s
9. 5-chloro-n-({(5s)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide
10. Chebi:68579
11. Jnj39039039
12. Jnj-39039039
13. 5-chloro-n-(((5s)-2-oxo-3-(4-(3-oxomorpholin-4-yl)phenyl)-1,3-oxazolidin-5-yl)methyl)thiophene-2-carboxamide
14. 5-chloro-n-{[(5s)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl}thiophene-2-carboxamide
15. Rivaroxaban (xarelto)
16. 2-thiophenecarboxamide, 5-chloro-n-(((5s)-2-oxo-3-(4-(3-oxo-4-morpholinyl)phenyl)-5-oxazolidinyl)methyl)-
17. 5-chloro-n-[[(5s)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl]thiophene-2-carboxamide
18. Xarelto (tn)
19. Rivaroxaban [inn]
20. Unii-9ndf7jz4m3
21. Hsdb 8149
22. Riv
23. Rivaroxaban [usan:inn:ban:jan]
24. Rivaroxaban- Bio-x
25. Rivaroxaban [mi]
26. Rivaroxaban [jan]
27. Rivaroxaban [usan]
28. Rivaroxaban [vandf]
29. Schembl3914
30. Rivaroxaban [mart.]
31. Rivaroxaban [usp-rs]
32. Rivaroxaban [who-dd]
33. Mls006010027
34. Rivaroxaban (jan/usan/inn)
35. Rivaroxaban [ema Epar]
36. Bdbm7840
37. Chembl198362
38. Gtpl6388
39. Dtxsid3057723
40. Amy1799
41. Ex-a206
42. Rivaroxaban [orange Book]
43. 2w26
44. Rivaroxaban [ep Monograph]
45. Rivaroxaban Enantiomer (r-isomer)
46. Zinc3964126
47. Rivaroxaban,xarelto,bay 59-7939
48. Mfcd11974010
49. Akos005145918
50. Ccg-212899
51. Cs-0555
52. Db06228
53. Ncgc00262945-10
54. Ncgc00379033-04
55. Br164355
56. Hy-50903
57. Smr002529611
58. A14979
59. D07086
60. Ab01563270_01
61. Us8822458, 44
62. Us8822458, 97
63. 789r028
64. Q420262
65. Sr-01000944189
66. Q-102503
67. Sr-01000944189-1
68. Brd-k37130656-001-01-2
69. (s)-5-chloro-n-((2-oxo-3-(4-(3-oxomorpholino)phenyl)-oxazolidin-5-yl)methyl )thiophene-2-carboxamide
70. (s)-5-chloro-n-((2-oxo-3-(4-(3-oxomorpholino)phenyl)-oxazolidin-5-yl)methyl)thiophene-2-carboxamide
71. 1429742-50-6
72. 5-chlor-n-({(5s)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophenecarboxamide
73. 5-chloro-n-({(5s)-2-oxo-3-[4-(3-oxo-4-morpholinyl) Phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophenecarboxamide
74. 5-chloro-n-({(5s)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}-methyl)-2-thiophenecarboxamide
75. 5-chloro-n-({(5s)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophencarboxamide
76. 5-chloro-n-({(5s)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophene-carboxamide
77. 5-chloro-n-({(5s)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophenecarboxamide
78. 5-chloro-n-({(5s)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl} Methyl)thiophene-2-carboxamide
79. 5-chloro-n-[[(5s)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-2-thiophenecarboxamide
80. 5-chloro-n-[[(s)-3-(4-(3-oxomorpholin-4-yl)phenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]-thiophene-2-carboxamide
81. 5-chloro-n-{[(5s)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]oxazolidin-5-yl]methyl}thiophene-2-carboxamide
82. R-rivaroxaban; Ent-rivaroxaban; [({(5r)-2-oxo-3-[p-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)amino](5-chloro-2-thienyl)formaldehyde; 5-chloro-n-[[(5r)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-2-thiophenecarboxamide
83. Rivaroxaban; (s)-5-chloro-n-((2-oxo-3-(4-(3-oxomorpholino)phenyl)oxazolidin-5-yl)methyl)thiophene-2-carboxamide
| Molecular Weight | 435.9 g/mol |
|---|---|
| Molecular Formula | C19H18ClN3O5S |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 435.0655696 g/mol |
| Monoisotopic Mass | 435.0655696 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 645 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Xarelto |
| PubMed Health | Rivaroxaban (By mouth) |
| Drug Classes | Anticoagulant |
| Drug Label | Rivaroxaban, a FXa inhibitor, is the active ingredient in XARELTO Tablets with the chemical name 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophenecarboxamide. The molecular formula of rivaroxaban is C19... |
| Active Ingredient | Rivaroxaban |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 15mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Xarelto |
| PubMed Health | Rivaroxaban (By mouth) |
| Drug Classes | Anticoagulant |
| Drug Label | Rivaroxaban, a FXa inhibitor, is the active ingredient in XARELTO Tablets with the chemical name 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-thiophenecarboxamide. The molecular formula of rivaroxaban is C19... |
| Active Ingredient | Rivaroxaban |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 15mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
Anticoagulant
National Library of Medicine's Medical Subject Headings. Rivaroxaban. Online file (MeSH, 2014). Available from, as of November 19, 2013: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Xarelto is indicated for the treatment of deep vein thrombosis (DVT). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
Xarelto is indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. There are limited data on the relative effectiveness of Xarelto and warfarin in reducing the risk of stroke and systemic embolism when warfarin therapy is well-controlled. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
Xarelto is indicated for the prophylaxis of deep vein thrombosis, which may lead to pulmonary embolism in patients undergoing knee or hip replacement surgery. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
For more Therapeutic Uses (Complete) data for Rivaroxaban (7 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: PREMATURE DISCONTINUATION OF XARELTO INCREASES THE RISK OF THROMBOTIC EVENTS. Premature discontinuation of any oral anticoagulant, including Xarelto, increases the risk of thrombotic events. If anticoagulation with Xarelto is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
/BOXED WARNING/ WARNING: SPINAL/EPIDURAL HEMATOMA. Epidural or spinal hematomas have occurred in patients treated with Xarelto who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include: use of indwelling epidural catheters; concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants; a history of traumatic or repeated epidural or spinal punctures a history of spinal deformity or spinal surgery. Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
Rivaroxaban increases the risk of hemorrhage and can cause serious or fatal bleeding. Bleeding complications were the most common adverse effects of rivaroxaban reported in clinical trials.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1472
Use of rivaroxaban should be avoided in patients with moderate (Child-Pugh class B) or severe (Child-Pugh class C) hepatic impairment or with any hepatic disease associated with coagulopathy; systemic exposure and risk of bleeding may be increased in such patients.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1471
For more Drug Warnings (Complete) data for Rivaroxaban (13 total), please visit the HSDB record page.
Rivaroxaban is indicated for the prevention of venous thromboembolic events (VTE) in patients who have undergone total hips replacements and total knee replacement surgery; prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation; treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE); to reduce risk of recurrent DVT and/or PE. Rivaroxaban is also indicated, in combination with aspirin, for reducing the risk of major cardiovascular events in patients with chronic coronary artery disease or peripheral artery disease. Its use is also not recommended in those with severe renal impairment (<30mL/min). Rivaroxaban is also indicated for the treatment and prevention of VTE in pediatric patients (from birth to 18 years of age) and for thromboprophylaxis in pediatric patients 2 years old with congenital heart disease following the Fontan procedure.
FDA Label
Xarelto, co-administered with acetylsalicylic acid (ASA) alone or with ASA plus clopidogrel or ticlopidine, is indicated for the prevention of atherothrombotic events in adult patients after an acute coronary syndrome (ACS) with elevated cardiac biomarkers.
Xarelto, co-administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with coronary artery disease (CAD) or symptomatic peripheral artery disease (PAD) at high risk of ischaemic events.
Prevention of venous thromboembolism (VTE) in adult patients undergoing elective hip or knee replacement surgery.
Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.
Adults
Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age 75 years, diabetes mellitus, prior stroke or transient ischaemic attack.
Paediatric population
Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing from 30 kg to 50 kg after at least 5 days of initial parenteral anticoagulation treatment.
Paediatric population
Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing more than 50 kg after at least 5 days of initial parenteral anticoagulation treatment.
Prevention of thromboembolic events, Treatment of thromboembolic events
Prevention of venous thromboembolism (VTE) in adult patients undergoing elective hip or knee replacement surgery.
Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. (See section 4. 4 for haemodynamically unstable PE patients.
Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. (See section 4. 4 for haemodynamically unstable PE patients).
* Adults:
Prevention of stroke and systemic embolism in adult patients with non valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age 75 years, diabetes mellitus, prior stroke or transient ischaemic attack.
Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. (See section 4. 4 for haemodynamically unstable PE patients. )
* Paediatric population:
Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing from 30 kg to 50 kg after at least 5 days of initial parenteral anticoagulation treatment.
Rivaroxaban Accord, co administered with acetylsalicylic acid (ASA) alone or with ASA plus ticlopidine, is indicated for the prevention of atherothrombotic events in adult patients after an acute coronary syndrome (ACS) with elevated cardiac biomarkers (see sections 4. 3, 4. 4 and 5. 1).
Rivaroxaban Accord, co administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with coronary artery disease (CAD) or symptomatic peripheral artery disease (PAD) at high risk of ischaemic events.
* Adults:
Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age 75 years, diabetes mellitus, prior stroke or transient ischaemic attack.
Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. (See section 4. 4 for haemodynamically unstable PE patients. )
* Paediatric population:
Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing more than 50 kg after at least 5 days of initial parenteral anticoagulation treatment.
Rivaroxaban Mylan co-administered with acetylsalicylic acid (ASA) alone or with ASA plus clopidogrel or ticlopidine, is indicated for the prevention of atherothrombotic events in adult patients after an acute coronary syndrome (ACS) with elevated cardiac biomarkers.
Rivaroxaban Mylan co-administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with coronary artery disease (CAD) or symptomatic peripheral artery disease (PAD) at high risk of ischaemic events.
------
Prevention of venous thromboembolism (VTE) in adult patients undergoing elective hip or knee replacement surgery.
Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.
-------
* Adults:
Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age 75 years, diabetes mellitus, prior stroke or transient ischaemic attack.
* Paediatric population:
Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing from 30 kg to 50 kg after at least 5 days of initial parenteral anticoagulation treatment.
* Paediatric population:
Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing more than 50 kg after at least 5 days of initial parenteral anticoagulation treatment.
Rivaroxaban is an anticoagulant which binds directly to factor Xa. Thereafter, it effectively blocks the amplification of the coagulation cascade, preventing the formation of thrombus. Rivaroxaban is a unqiue anticoagulant for two reasons. First of all, it is does not involve antithrombin III (ATIII) to exert its anticoagulant effects. Secondly, it is an oral agent whereas the widely used unfractionated heparin and low molecular weight heparins are for parenteral use only. Although the activated partial thromboplastin time (aPTT) and HepTest (a test developed to assay low molecular weight heparins) are prolonged in a dose-dependant manner, neither test is recommended for the assessment of the pharmacodynamic effects of rivaroxaban. Anti-Xa activity and inhibition of anti-Xa activity monitoring is also not recommended despite being influenced by rivaroxaban.
Factor Xa Inhibitors
Endogenous factors and drugs that inhibit or block the activity of FACTOR XA. (See all compounds classified as Factor Xa Inhibitors.)
B01AF01
B01AF01
B01AF01
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AF - Direct factor xa inhibitors
B01AF01 - Rivaroxaban
Absorption
Following oral administration, rivaroxaban is rapidly absorbed and reaches peak plasma concentration in 2-4 hours. Bioavailability of the 10 mg dose is >80%. However, the 15-20 mg dose have a lower bioavailability if taken in the fasted state and consequently should be taken with food.
Route of Elimination
Approximately two-thirds of rivaroxaban is excreted into urine (via active tubular secretion in which approximately 36% as unchanged drug and 30% as inactive metabolism). The remaining third of the administered dose is excreted via feces in which 7% is in the form of unchanged drug and 21% as inactive metabolites.
Volume of Distribution
The steady state Vd is 50 L
Clearance
Systemic clearance is approximately 10 L/h, so rivaroxaban is considered a drug with low clearance. Renal clearance is ~3-4 L/h.
Following oral administration, approximately one-third of the absorbed dose is excreted unchanged in the urine, with the remaining two-thirds excreted as inactive metabolites in both the urine and feces. In a Phase 1 study, following the administration of a (14)C-rivaroxaban dose, 66% of the radioactive dose was recovered in urine (36% as unchanged drug) and 28% was recovered in feces (7% as unchanged drug). Unchanged drug is excreted into urine, mainly via active tubular secretion and to a lesser extent via glomerular filtration (approximate 5:1 ratio). Rivaroxaban is a substrate of the efflux transporter proteins P-gp and ABCG2 (also abbreviated Bcrp). Rivaroxaban's affinity for influx transporter proteins is unknown.
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
Plasma protein binding of rivaroxaban in human plasma is approximately 92% to 95%, with albumin being the main binding component. The steady-state volume of distribution in healthy subjects is approximately 50 L.
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
Absorption of rivaroxaban is dependent on the site of drug release in the GI tract. A 29% and 56% decrease in AUC and Cmax compared to tablet was reported when rivaroxaban granulate is released in the proximal small intestine. Exposure is further reduced when drug is released in the distal small intestine, or ascending colon. Avoid administration of rivaroxaban distal to the stomach which can result in reduced absorption and related drug exposure.
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
The absolute bioavailability of rivaroxaban is dose-dependent. For the 10 mg dose, it is estimated to be 80% to 100% and is not affected by food. Xarelto 10 mg tablets can be taken with or without food. For the 20 mg dose in the fasted state, the absolute bioavailability is approximately 66%. Coadministration of Xarelto with food increases the bioavailability of the 20 mg dose (mean AUC and Cmax increasing by 39% and 76% respectively with food). Xarelto 15 mg and 20 mg tablets should be taken with food.
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
For more Absorption, Distribution and Excretion (Complete) data for Rivaroxaban (8 total), please visit the HSDB record page.
Approximately two-thirds of the dose is metabolized. It is metabolized by CYP3A4, CYP3A5, CYP2J2 and CYP-independant mechanisms
Rivaroxaban undergoes oxidative degradation by cytochrome P-450 (CYP) isoenzymes 3A4/5 and 2J2 and hydrolysis; metabolites are subsequently eliminated through renal and fecal/biliary routes. No major circulating metabolites have been identified in plasma.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1474
The terminal half life is 5-9 hours in adults and 11-13 hours in the elderly.
The terminal elimination half-life is 11 to 13 hours in the elderly.
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610
Rivaroxaban competitively inhibits free and clot bound factor Xa. Factor Xa is needed to activate prothrombin (factor II) to thrombin (factor IIa). Thrombin is a serine protease that is required to activate fibrinogen to fibrin, which is the loose meshwork that completes the clotting process. Since one molecule of factor Xa can generate more than 1000 molecules of thrombin, selective inhibitors of factor Xa are profoundly useful in terminating the amplification of thrombin generation. The action of rivaroxaban is irreversible.
Rivaroxaban, an oral, direct activated factor X (Xa) inhibitor, is an anticoagulant. Factor Xa plays a central role in the blood coagulation cascade by serving as the convergence point for the intrinsic and extrinsic pathways; inhibition of coagulation factor Xa by rivaroxaban prevents conversion of prothrombin to thrombin and subsequent thrombus formation. Rivaroxaban inhibits both free and prothrombinase-bound factor Xa. Unlike fondaparinux, heparin, and the low molecular weight heparins, rivaroxaban binds directly to the active site of factor Xa without the need for a cofactor (e.g., antithrombin III). Rivaroxaban inhibits factor Xa with more than 100,000-fold greater selectivity than other biologically important serine proteases (e.g., thrombin, trypsin, plasmin, factor VIIa, factor IXa, urokinase, activated protein C).
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1474
Xarelto is an orally bioavailable factor Xa inhibitor that selectively blocks the active site of factor Xa and does not require a cofactor (such as Anti-thrombin III) for activity. Activation of factor X to factor Xa (FXa) via the intrinsic and extrinsic pathways plays a central role in the cascade of blood coagulation.
US Natl Inst Health; DailyMed. Current Medication Information for XARELTO (rivaroxaban) tablet, film coated (August 2013). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=10db92f9-2300-4a80-836b-673e1ae91610