
1. Mk 0966
2. Mk 966
3. Mk-0966
4. Mk-966
5. Refecoxib
6. Vioxx
7. Vioxx Dolor
1. 162011-90-7
2. Vioxx
3. Ceoxx
4. Mk 966
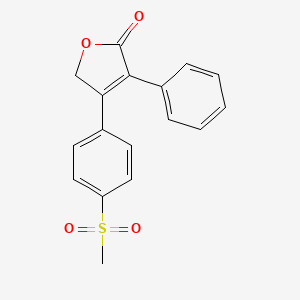
5. 4-(4-(methylsulfonyl)phenyl)-3-phenylfuran-2(5h)-one
6. 4-[4-(methylsulfonyl)phenyl]-3-phenylfuran-2(5h)-one
7. Mk-966
8. Mk-0966
9. 4-[4-(methylsulfonyl)phenyl]-3-phenyl-2(5h)-furanone
10. Mk0966
11. Mk 0966
12. 3-(4-methylsulfonylphenyl)-4-phenyl-2h-furan-5-one
13. Rofecoxib (vioxx)
14. Trm-201
15. 3-phenyl-4-[4-(methylsulfonyl)phenyl]-2(5h)-furanone
16. 4-(4-(methylsulfonyl)phenyl)-3-phenyl-2(5h)-furanone
17. 0qtw8z7mcr
18. Chembl122
19. Nsc-720256
20. Nsc-758705
21. 4-(p-(methylsulfonyl)phenyl)-3-phenyl-2(5h)-furanone
22. 2(5h)-furanone, 4-[4-(methylsulfonyl)phenyl]-3-phenyl-
23. Chebi:8887
24. M01ah02
25. Mk966
26. Refecoxib
27. Ncgc00095118-01
28. 4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofuran-2-one
29. Vioxx Dolor
30. 2(5h)-furanone, 4-(4-(methylsulfonyl)phenyl)-3-phenyl-
31. Vioxx (trademark)
32. Smr000466331
33. Ccris 8967
34. Vioxx (tn)
35. Hsdb 7262
36. Sr-01000762904
37. Unii-0qtw8z7mcr
38. Rofecoxibum
39. Rofecoxib (jan/usan/inn)
40. Rofecoxib [usan:inn:ban]
41. 3-phenyl-4-(4-(methylsulfonyl)phenyl))-2(5h)-furanone
42. Rofecoxib [usan]
43. 4-(4-methylsulfonylphenyl)-3-phenyl-5h-furan-2-one
44. Ks-1107
45. Mk 0996
46. Spectrum_000119
47. Rofecoxib [inn]
48. Rofecoxib [jan]
49. Specplus_000669
50. Rofecoxib [mi]
51. Rofecoxib [hsdb]
52. Spectrum2_000446
53. Spectrum3_001153
54. Spectrum4_000631
55. Spectrum5_001598
56. Rofecoxib [vandf]
57. Dsstox_cid_3567
58. Rofecoxib [mart.]
59. Rofecoxib [who-dd]
60. Schembl3050
61. Dsstox_rid_77084
62. Dsstox_gsid_23567
63. Bspbio_002705
64. Kbiogr_001242
65. Kbiogr_002345
66. Kbioss_000559
67. Kbioss_002348
68. Mls000759440
69. Mls001165770
70. Mls001195623
71. Mls001424113
72. Mls006010091
73. Bidd:gt0399
74. Divk1c_006765
75. Spectrum1504235
76. Spbio_000492
77. 3-(4-methanesulfonylphenyl)-2-phenyl-2-buten-4-olide
78. Gtpl2893
79. Trm201
80. Zinc7455
81. Rofecoxib [orange Book]
82. Dtxsid2023567
83. Bdbm22369
84. Kbio1_001709
85. Kbio2_000559
86. Kbio2_002345
87. Kbio2_003127
88. Kbio2_004913
89. Kbio2_005695
90. Kbio2_007481
91. Kbio3_002205
92. Kbio3_002825
93. Ex-a708
94. Cmap_000024
95. Hms1922h11
96. Hms2051g16
97. Hms2089h20
98. Hms2093e04
99. Hms2232g21
100. Hms3371p11
101. Hms3393g16
102. Hms3651f16
103. Hms3713b07
104. Hms3750i17
105. Hms3885e05
106. Pharmakon1600-01504235
107. Bcp03619
108. Tox21_111430
109. Ccg-40253
110. Mfcd00935806
111. Nsc720256
112. Nsc758705
113. S3043
114. Stk635144
115. Akos000280931
116. Ab07701
117. Cs-0997
118. Db00533
119. Nc00132
120. Nsc 720256
121. Nsc 758705
122. Sb19518
123. Ncgc00095118-02
124. Ncgc00095118-03
125. Ncgc00095118-04
126. Ncgc00095118-05
127. Ncgc00095118-08
128. Ncgc00095118-17
129. Ncgc00095118-18
130. Ac-28318
131. Br164362
132. Hy-17372
133. Nci60_041175
134. Sbi-0206774.p001
135. Cas-162011-90-7
136. Ft-0631192
137. R0206
138. Sw219668-1
139. C07590
140. D00568
141. Ab00052090-06
142. Ab00052090-08
143. Ab00052090_09
144. Ab00052090_10
145. 011r907
146. A810324
147. L000912
148. Q411412
149. Q-201676
150. Sr-01000762904-3
151. Sr-01000762904-5
152. Brd-k21733600-001-02-6
153. Brd-k21733600-001-06-7
154. 3-(4-methanesulfonyl-phenyl)-2-phenyl-2-buten-4-olide
155. 2(5h)-furanone, 4-[4-(methyl-sulfonyl)phenyl]-3-phenyl-
156. 3-(phenyl)-4-(4-(methylsulfonyl)phenyl)-2-(5h)-furanone
157. 3-phenyl-4-(4-(methylsulfonyl)phenyl)-2-(5h)-furanone
158. 4-(4-methanesulfonyl-phenyl)-3-phenyl-5h-furan-2-one
159. 4-(4-methylsulfonylphenyl)-3-phenyl-2,5-dihydro-2-furanone
| Molecular Weight | 314.4 g/mol |
|---|---|
| Molecular Formula | C17H14O4S |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 314.06128010 g/mol |
| Monoisotopic Mass | 314.06128010 g/mol |
| Topological Polar Surface Area | 68.8 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 556 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/September 20, 2004/ Merck & Co., Inc. announced a voluntary withdrawal of Vioxx (rofecoxib) from the U.S. and worldwide market due to safety concerns of an increased risk of cardiovascular events (including heart attack and stroke) in patients on Vioxx. Vioxx is a prescription COX-2 selective, non-steroidal anti-inflammatory drug (NSAID) that was approved by FDA in May 1999 for the relief of the signs and symptoms of osteoarthritis, for the management of acute pain in adults, and for the treatment of menstrual symptoms, and was later approved for the relief of the signs and symptoms of rheumatoid arthritis in adults and children.
US FDA; MedWatch. The FDA Safety Information and Adverse Event Reprting Program. 2004 Safety Alerts for Drugs, Biologics, Medical Devises, and Dietary Supplements. Vioxx (rofecoxib). Washington, DC: Fodd Drug Admin. Available from, as of Oct 20, 2004: https://www.fda.gov/medwatch/SAFETY/2004/safety04.htm#vioxx
Anti-inflammatory.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1481
Rofecoxib is indicated for the relief of the signs and symptoms of osteoarthritis. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2495
Rofecoxib is indicated for short-term use (5 days) for relief of acute pain, especially when anti-inflammatory actions may be desired, such as following dental or orthopedic surgery. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2495
Rofecoxib is indicated for short-term use (5 days) for relief of the pain and other symptoms of primary dysmenorrhea. /Included in US product labeling
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2495
/Merck & Co., Inc. announced a voluntary withdrawal of Vioxx (rofecoxib) from the U.S. and worldwide market/ due to safety concerns of an increased risk of cardiovascular events (including heart attack and stroke) in patients on Vioxx. Vioxx is a prescription COX-2 selective, non-steroidal anti-inflammatory drug (NSAID) that was approved by FDA in May 1999 for the relief of the signs and symptoms of osteoarthritis, for the management of acute pain in adults, and for the treatment of menstrual symptoms, and was later approved for the relief of the signs and symptoms of rheumatoid arthritis in adults and children.
US FDA; MedWatch. The FDA Safety Information and Adverse Event Reprting Program. 2004 Safety Alerts for Drugs, Biologics, Medical Devises, and Dietary Supplements. Vioxx (rofecoxib). Washington, DC: Fodd Drug Admin. Available from, as of Oct 20, 2004: https://www.fda.gov/medwatch/SAFETY/2004/safety04.htm#vioxx
There is controversy whether cyclooxygenase-2 (COX-2) specific inhibitors are associated with elevations in blood pressure requiring treatment in typical clinical practice. We examined the risk of new onset hypertension in a retrospective case-control study involving 17 844 subjects aged > or =65 years from 2 US states. Multivariable logistic models were examined to assess the relative risk of new onset hypertension requiring treatment in patients who used celecoxib or rofecoxib compared with patients taking either the other COX-2 specific inhibitor, a nonspecific NSAID, or no NSAID. During the 1999 to 2000 study period, 3915 patients were diagnosed and began treatment for hypertension; 4 controls were selected for every case. In no model was celecoxib significantly associated with the development of hypertension. Rofecoxib users were at a significantly increased relative risk of new onset hypertension compared with patients taking celecoxib (odds ratio (OR) 1.6; 95% confidence interval (CI), 1.2 to 2.1), taking a nonspecific NSAID (OR 1.4; 95% CI, 1.1 to 1.9), or taking no NSAID (OR 1.6; 95% CI, 1.3 to 2.0). There were no clear dosage or duration effects. In patients with a history of chronic renal disease, liver disease, or congestive heart failure, the relative risk of new onset hypertension was twice as high in those taking rofecoxib compared with celecoxib (OR 2.1; 95% CI, 1.0 to 4.3). In this retrospective case-control study of patients aged > or =65 years, rofecoxib use was associated with an increased relative risk of new onset hypertension; this was not seen in patients taking celecoxib.
PMID:15226279 Solomon DH et al; Hypertension 44 (2): 140-5 (2004)
In a double-blind study, 35 stable subjects on low-dose aspirin with > or = 2 previous acute coronary events and 2 of 2 screening CRP values >2.0 mg/L were randomized to the COX-2 inhibitor rofecoxib (25 mg) or placebo daily for 6 months. Serum CRP, interleukin-6 (IL-6), P-selectin, matrix metalloproteinase-9 (MMP-9), and brachial artery endothelial function were evaluated. In the placebo group, CRP (median) was 3.16 mg/L (25% and 75% quartiles, 1.90 and 5.78 mg/L) at baseline and 4.22 mg/L (25% and 75% quartiles, 2.04 and 6.25 mg/L) at 6 months; in the rofecoxib group, CRP was 3.45 mg/L (25% and 75% quartiles, 2.08 and 5.78 mg/L) at baseline and 1.41 mg/L (25% and 75% quartiles, 1.17 and 4.81 mg/L) at 6 months (P=0.03). Rofecoxib compared with placebo also lowered IL-6 at 6 months (P=0.0002). There was a significant off-drug effect on CRP and IL-6 levels in the rofecoxib group 3 months after treatment (P=0.005 and P=0.009, respectively). Rofecoxib did not significantly affect P-selectin, MMP-9, and brachial artery vasoreactivity. Prolonged COX-2 inhibition attenuates CRP and IL-6, does not modify P-selectin and MMP-9, and has no deleterious effect on endothelial function in stable patients with a history of recurrent acute coronary events and raised CRP. These results strengthen the rationale for evaluating the clinical benefit of COX-2 inhibition in patients with ischemic heart disease.
PMID:15302800 Bogaty P et al; Circulation 110 (8): 934-9 (2004)
A 73-year-old woman was prescribed rofecoxib 25 mg/day for rheumatoid arthritis in addition to other medications on which the patient had been stabilized. Six months after initiation of rofecoxib, linear plaques over the infra-orbital and bitemporal areas of both eyes were observed. Several itchy violaceous papules also developed on her right wrist and dorsum of the left foot. She also had a hyperpigmented macule on her right buccal mucosa. As the skin rash was localized and the patient was initially unwilling to undergo skin biopsy, rofecoxib was continued and a topical steroid was started. One month later, the patient was seen in the dermatology clinic, and the improvement of her skin reaction was significant. A skin biopsy performed during this visit was consistent with LDE. On the next day, her rheumatologist decided to discontinue the offending drug, rofecoxib. Two months later, all skin lesions had completely resolved. No rechallenge with rofecoxib was attempted. LDE is a rare skin reaction that can be associated with several drugs. Rofecoxib, a cyclooxygenase-2 inhibitor, has never before been reported to cause LDE. An objective causality assessment indicates that rofecoxib was the probable cause of the skin reaction.
PMID:15026562 Abu-Shraie NA, Alfadley AA; Ann Pharmacother 38 (5): 795-8 (2004)
For more Drug Warnings (Complete) data for ROFECOXIB (31 total), please visit the HSDB record page.
For the treatment of osteoarthritis, rheumatoid arthritis, acute pain in adults, and primary dysmenorrhea, as well as acute treatment of migraine attacks with or without auras.
FDA Label
Rofecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, is classified as a nonsteroidal anti-inflammatory drug (NSAID). Unlike celecoxib, rofecoxib lacks a sulfonamide chain and does not require CYP450 enzymes for metabolism. Like other NSAIDs, rofecoxib exhibits anti-inflammatory, analgesic, and antipyretic activity. NSAIDs appear to inhibit prostaglandin synthesis via the inhibition of cyclooxygenase (COX), which are responsible for catalyzing the formation of prostaglandins in the arachidonic acid pathyway. There are at least two isoenzymes, COX-1 and COX-2, that have been identified. Although the exact mechanisms have not been clearly established, NSAIDs exert their anti-inflammatory, analgesic, and antipyretic primarily through the inhibition of COX-2. The inhibition of COX-1 is principally responsible for the negative effects on the GI mucosa. As rofecoxib is selective for COX-2, it may be potentially associated with a decreased risk of certain adverse events, but more data is needed to fully evaulate the drug.
Cyclooxygenase 2 Inhibitors
A subclass of cyclooxygenase inhibitors with specificity for CYCLOOXYGENASE-2. (See all compounds classified as Cyclooxygenase 2 Inhibitors.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AH - Coxibs
M01AH02 - Rofecoxib
Absorption
The mean oral bioavailability of rofecoxib at therapeutically recommended doses of 12.5, 25, and 50 mg is approximately 93%.
Approximately 72% and 14% of a radioactive rofecoxib dose is eliminated in the urine as metabolites and /in/ feces as unchanged drug, respectively.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2496
Time to peak concentration: Approximately 2 to 3 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2496
At steady state, the apparent volume of distribution is about 91 and 86 L after a 12.5 and 25 mg dose, respectively.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2495
At recommended doses, the mean oral bioavailability is 93%. The peak plasma concentration and area under the plasma concentration-time curve are roughly proportional across the clinical dose range.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2495
For more Absorption, Distribution and Excretion (Complete) data for ROFECOXIB (11 total), please visit the HSDB record page.
Hepatic. Metabolism of rofecoxib is primarily mediated through reduction by cytosolic enzymes. The principal metabolic products are the cis-dihydro and trans-dihydro derivatives of rofecoxib, which account for nearly 56% of recovered radioactivity in the urine. An additional 8.8% of the dose was recovered as the glucuronide of the hydroxy derivative, a product of oxidative metabolism. The biotransformation of rofecoxib and this metabolite is reversible in humans to a limited extent (< 5%). These metabolites are inactive as COX-1 or COX-2 inhibitors. Cytochrome P450 plays a minor role in metabolism of rofecoxib.
The metabolism of rofecoxib, a potent and selective inhibitor of cyclooxygenase-2, was examined in vitro using human liver subcellular fractions. The biotransformation of rofecoxib was highly dependent on the subcellular fraction and the redox system used. In liver microsomal incubations, NADPH-dependent oxidation of rofecoxib to 5-hydroxyrofecoxib predominated, whereas NADPH-dependent reduction of rofecoxib to the 3,4-dihydrohydroxy acid metabolites predominated in cytosolic incubations. In incubations with S9 fractions, metabolites resulting from both oxidative and reductive pathways were observed. In contrast to microsomes, the oxidation of rofecoxib to 5-hydroxyrofecoxib by S9 fractions followed two pathways, one NADPH-dependent and one NAD+-dependent (non-cytochrome P450), with the latter accounting for about 40% of total activity. The 5-hydroxyrofecoxib thus formed was found to undergo NADPH-dependent reduction ("back reduction") to rofecoxib in incubations with liver cytosolic fractions. In incubations with dialyzed liver cytosol, net hydration of rofecoxib to form 3,4-dihydro-5-hydroxyrofecoxib was observed, whereas the 3,4-dihydrohydroxy acid derivatives were formed when NADPH was present. Although 3,4-dihydro-5-hydroxyrofecoxib could be reduced to the 3,4-dihydrohydroxy acid by cytosol in the presence of NADPH, the former species does not appear to serve as an intermediate in the overall reductive pathway of rofecoxib metabolism. In incubations of greater than 2 h with S9 fractions, net reductive metabolism predominated over oxidative metabolism. These in vitro results are consistent with previous findings on the metabolism of rofecoxib in vivo in human and provide a valuable insight into mechanistic aspects of the complex metabolism of this drug.
PMID:14570773 Slaughter D et al; Drug Metab Dispos 31 (11): 1398-408 (2003)
Metabolism of rofecoxib is primarily mediated through reduction by cytosolic enzymes. The principal metabolic products are the cis-dihydro and trans-dihydro derivatives of rofecoxib, which account for nearly 56% of recovered radioactivity in the urine. An additional 8.8% of the dose was recovered as the glucuronide of the hydroxy derivative, a product of oxidative metabolism. The biotransformation of rofecoxib and this metabolite is reversible in humans to a limited extent (<5%). Theses metabolites are inactive as COX-1 or COX-2 inhibitors.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 2108
Rofecoxib has known human metabolites that include 5-hydroxy-rofecoxib.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
17 hours
Approximately 17 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2495
The anti-inflammatory, analgesic, and antipyretic effects of NSAIDs appear to result from the inhibition of prostaglandin synthesis. Although the exact mechanism of action has not been determined, these effects appear to be mediated through the inhibition of the COX-2 isoenzyme at the sites of inflammation with subsequent reduction in the synthesis of certain prostaglandins from their arachidonic acid precursors. Rofecoxib selectively inhibits the cyclooxygenase-2 (COX-2) enzyme, which is important for the mediation of inflammation and pain. Unlike non-selective NSAIDs, rofecoxib does not inhibit platelet aggregation. It also has little to no affinity for COX-1.
Rofecoxib is a nonsteroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic, and antipyretic therapeutic effects. It has been proposed that rofecoxib inhibits the activity of the enzyme cyclooxygenase-2 (COX-2), resulting in a decreased formation of precursors of prostaglandins. Rofecoxib does not inhibit cyclooxygenase-1 (COX-1) isoenzyme in humans at therapeutic concentrations.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2495