

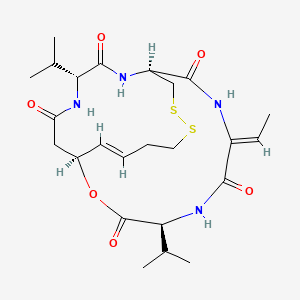

1. Fk228
2. Fr 901228
3. Fr-901228
4. Fr901228
5. L-valine, N-(3-hydroxy-7-mercapto-1-oxo-4-heptenyl)-d-valyl-d-cysteinyl-(z)-2,3-didehydro-2-aminobutanoyl-, Xi-lactone, Cyclic (1-2)-disulfide, (s-(e))-
6. Romidepsin
1. Romidepsin
2. Depsipeptide
3. Fk228
4. Chromadax
5. 128517-07-7
6. Antibiotic Fr 901228
7. Fr901228
8. Fk 228
9. Fk-228
10. Fr 901228
11. Fr-901228
12. Nsc-630176
13. Chembl343448
14. (1s,4s,7z,10s,16e,21r)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
15. Chebi:61080
16. Nsc 630176
17. Nsc630176
18. Nsc754143
19. (1s,4s,7z,10s,16e,21r)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
20. C24h36n4o6s2
21. (1s,4s,7z,10s,16e,21r)-7-ethylidene-4,21-bis(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
22. Istodax (tn)
23. Romidepsin [usan]
24. Cx3t89xqbk
25. (1s,4s,7z,10s,16e,21r)-7-ethylidene-4,21-bis(1-methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo(8.7.6)tricos-16-ene-3,6,9,19,22-pentone
26. Hdinhib_000006
27. Romidepsina
28. Romidepsine
29. Romidepsinum
30. Oxa-12,8,20,23-tetrazabicyclo[8.7.6]tricosane, Cyclic Peptide Deriv.
31. Romidepsin (fk228)
32. Romidepsin; Fk-228
33. Romidepsin [mi]
34. Fk-901228
35. Romidepsin [inn]
36. Romidepsin [jan]
37. Romidepsin [vandf]
38. Probes1_000153
39. Probes2_000337
40. Romidepsin [mart.]
41. Romidepsin [who-dd]
42. Depsipeptide [who-dd]
43. Romidepsin (jan/usan/inn)
44. Schembl677497
45. Gtpl7006
46. Romidepsin [orange Book]
47. Romidepsin, >=98% (hplc)
48. Bdbm19151
49. Romidepsin (fk228 ,depsipeptide)
50. Cyclo((2z)-2-amino-2-butenoyl-l-valyl-(3s,4e)-3-hydroxy-7-mercapto-4-heptenoyl-d-valyl-d-cysteinyl), Cyclic (3->5)-disulfide
51. Zinc3935130
52. Mfcd18433404
53. S3020
54. Api0005301
55. Cs-0985
56. Db06176
57. Nsc-754143
58. Hy-15149
59. D06637
60. Ab01273968-01
61. Sr-01000941579
62. Q7363205
63. Sr-01000941579-1
64. L-valine,3-didehydro-2- Aminobutanoyl-,.xi.-lactone, Cyclic (1.fwdarw.2)-disulfide
65. L-valine,3-didehydro-2-aminobutanoyl-,.xi.-lactone, Cyclic (1.fwdarw.2)-disulfide
66. (1s,4s,7z,10s,16e,21r)-7- Ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23- Tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
67. (1s,4s,7z,10s,16e,21r)-7-ethylidene-4,21-bis(1methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16ene-3,6,9,19,22-pentone
68. (e)-(1s,10s,21r)-7-[(z)-ethylidene]-4,21-diisopropyl-2- Oxa-12,13-dithia-5,8,20,23- Tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
69. (e)-(1s,10s,21r)-7-[(z)-ethylidene]-4,21-diisopropyl-2- Oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
70. Cyclo((2z)-2-amino-2-butenoyl-l-valyl-(3s,4e)-3-hydroxy-7-mercapto-4-heptenoyl-d-valyl-d-cysteinyl), Cyclic (3->5)-disulphide
71. Cyclo[(2z)-2-amino-2-butenoyl-l-val Yl-(3s,4e)-3-hydroxy-7-mercapto-4-heptenoyl-d-valy L-d-cysteinyl], Cyclic (3-5) Disulfide
72. Cyclo[(2z)-2-amino-2-butenoyl-l-valyl-(3s,4e)-3-hydroxy-7-mercapto-4-heptenoyl-d-valyl-d-cysteinyl], Cyclic (3-5) Disulfide
| Molecular Weight | 540.7 g/mol |
|---|---|
| Molecular Formula | C24H36N4O6S2 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 540.20762723 g/mol |
| Monoisotopic Mass | 540.20762723 g/mol |
| Topological Polar Surface Area | 193 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 905 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of cutaneous T-cell lymphoma (CTCL) or/and peripheral T-cell lymphoma (PTCL) in patients who have received at least one prior systemic therapy. These indications are based on response rate. Clinical benefit such as improvement in overall survival has not been demonstrated.
FDA Label
treatment of peripheral T-cell lymphoma (PTCL),
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Histone Deacetylase Inhibitors
Compounds that inhibit HISTONE DEACETYLASES. This class of drugs may influence gene expression by increasing the level of acetylated HISTONES in specific CHROMATIN domains. (See all compounds classified as Histone Deacetylase Inhibitors.)
L01XX39
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XH - Histone deacetylase (hdac) inhibitors
L01XH02 - Romidepsin
Absorption
Romidepsin exhibited linear pharmacokinetics at standard doses.
Volume of Distribution
44.5L
Clearance
8.4L/h
Romidepsin undergoes extensive hepatic metabolism in vitro primarily by CYP3A4 with minor contribution from CYP3A5, CYP1A1, CYP2B6 and CYP2C19.
Approximately 3 hours
Romidepsin is a prodrug, where it becomes active once taken up into the cell. The active metabolite has a free thiol group, which interacts with zinc ions in the active site of class 1 and 2 HDAC enzymes, resulting in inhibition of its enzymatic activity. Certain tumors have over expressed HDACs and downregulated/mutated histone acetyltransferases. This imbalance of HDAC relative to histone acetyltransferase can lead to a decrease in regulatory genes, ensuing tumorigenesis. Inhibition of HDAC may restore normal gene expression in cancer cells and result in cell cycle arrest and apoptosis.