

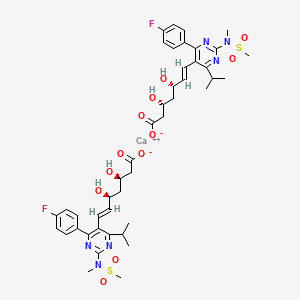

1. Calcium, Rosuvastatin
2. Crestor
3. Rosuvastatin
4. Zd 4522
5. Zd4522
1. 147098-20-2
2. Crestor
3. Rosuvastatin Hemicalcium
4. Fortius
5. Rostar
6. Rosuvastatin Calcium Salt
7. Rosuvastatin Calcium [usan]
8. Rozavel
9. Suvikan
10. Zd4522
11. S-4522
12. Zd 4522
13. Rosuvastatin (as Calcium)
14. Zd4522 (calcium Salt)
15. Zd4522 Calcium
16. Zd-4522 Calcium
17. 83mvu38m7q
18. Chebi:77249
19. Nsc-747274
20. Nsc-758930
21. Rosuvastatincalcium
22. Crestor (tn)
23. Calcium (3r,5s,e)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(n-methylmethylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoate
24. Rovista
25. Zd 4522, Calcium Salt
26. Calcium;(e,3r,5s)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate
27. S 4522
28. Unii-83mvu38m7q
29. Provisacor
30. Shufutan
31. Ezallor
32. Zd 4522 Calcium
33. Rosuvastatin Calcium- Bio-x
34. Zd-4522 (calcium Salt)
35. Schembl150740
36. Schembl429217
37. Chembl1744447
38. Dtxsid9045928
39. Rosuvastatin Calcium (jan/usan)
40. Rosuvastatin Calcium [jan]
41. Azd-4522
42. Bcp04131
43. Rosuvastatin Calcium [mart.]
44. S2169
45. Rosuvastatin Calcium [usp-rs]
46. Rosuvastatin Calcium [who-dd]
47. Akos005145896
48. Akos017343682
49. Rosuvastatin Calcium Salt [mi]
50. Ccg-270606
51. Ks-1109
52. Nsc 747274
53. Nsc 758930
54. (s-((r*,s*-(e)))- 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl) Amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic Acid, Calcium Salt (2:1)
55. (s-(r*,s*-(e)))-7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic Acid, Calcium Salt (2:1)
56. 6-heptenoic Acid, 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-, Calcium Salt (2:1), (3r,5s,6e)
57. As-12488
58. Br164378
59. Rosuvastatin Calcium [orange Book]
60. Rosuvastatin Calcium [ep Monograph]
61. Rosuvastatin Calcium [usp Monograph]
62. Roszet Component Rosuvastatin Calcium
63. R0180
64. D01915
65. Rosuvastatin Calcium Component Of Roszet
66. Q-201685
67. Q27146836
68. (s-((r*,s*-(e)))- 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl) Amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic Acid, Calcium Salt (2:1)
69. (s-(r*,s*-(e)))-7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic Acid, Calcium Salt
70. 6-heptenoic Acid, 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-, Calcium Salt (2:1), (3r,5s,6e)-
71. Bis((e)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(methyl(methylsulfonyl)amino)-pyrimide-5-yl)-(3r,5s)3,5-dihydroxylhept-6-enoic Acid) Calcium
72. Calcium Bis[(3r,5s,6e)-7-{4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl}-3,5-dihydroxyhept-6-enoate]
73. Calcium(3r,5s,e)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(n-methylmethylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoate
74. Monocalcium Bis((3r,5s,6e)-7-{4-(4-fluorophenyl)-6-isopropyl-2-[methanesulfonyl (methyl) Amino] Pyrimidin-5-yl}-3,5-dihydroxyhept-6-enoate)
1. Rosuvastatin
2. Crestor
3. X-plended
| Molecular Weight | 1001.1 g/mol |
|---|---|
| Molecular Formula | C44H54CaF2N6O12S2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 20 |
| Rotatable Bond Count | 18 |
| Exact Mass | 1000.2835107 g/mol |
| Monoisotopic Mass | 1000.2835107 g/mol |
| Topological Polar Surface Area | 304 Ų |
| Heavy Atom Count | 67 |
| Formal Charge | 0 |
| Complexity | 761 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 4 | |
|---|---|
| Drug Name | Crestor |
| Drug Label | CRESTOR (rosuvastatin calcium) is a synthetic lipid-lowering agent for oral administration. The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhe... |
| Active Ingredient | Rosuvastatin calcium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Ipr |
| 2 of 4 | |
|---|---|
| Drug Name | Rosuvastatin calcium |
| PubMed Health | Rosuvastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | CRESTOR (rosuvastatin calcium) is a synthetic lipid-lowering agent for oral administration. The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhe... |
| Active Ingredient | Rosuvastatin calcium |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 10mg; 40mg; 20mg |
| Market Status | Tentative Approval |
| Company | Mylan Pharma; Apotex; Aurobindo Pharma; Sandoz; Sun Pharma Global; Par Pharm; Watson Labs; Glenmark Generics; Teva Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Crestor |
| Drug Label | CRESTOR (rosuvastatin calcium) is a synthetic lipid-lowering agent for oral administration. The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhe... |
| Active Ingredient | Rosuvastatin calcium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Ipr |
| 4 of 4 | |
|---|---|
| Drug Name | Rosuvastatin calcium |
| PubMed Health | Rosuvastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Drug Label | CRESTOR (rosuvastatin calcium) is a synthetic lipid-lowering agent for oral administration. The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhe... |
| Active Ingredient | Rosuvastatin calcium |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 10mg; 40mg; 20mg |
| Market Status | Tentative Approval |
| Company | Mylan Pharma; Apotex; Aurobindo Pharma; Sandoz; Sun Pharma Global; Par Pharm; Watson Labs; Glenmark Generics; Teva Pharms |
Homozygous Familial Hypercholesterolaemia, Prevention of cardiovascular events, Primary combined (mixed) dyslipidaemia, Primary hypercholesterolaemia
Anticholesteremic Agents
Substances used to lower plasma cholesterol levels. (See all compounds classified as Anticholesteremic Agents.)
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Compounds that inhibit HYDROXYMETHYLGLUTARYL COA REDUCTASES. They have been shown to directly lower CHOLESTEROL synthesis. (See all compounds classified as Hydroxymethylglutaryl-CoA Reductase Inhibitors.)

