


1. Biaxsig
2. Claramid
3. Infectoroxit
4. Macrosil
5. Mtw-roxithromycin
6. Rotesan
7. Rotramin
8. Roxi 1a Pharma
9. Roxi Basics
10. Roxi Tad
11. Roxi Von Ct
12. Roxi-paed 1a Pharma
13. Roxi-puren
14. Roxi-q
15. Roxi-saar
16. Roxi-wolff
17. Roxibeta
18. Roxidura
19. Roxigamma
20. Roxigrn
21. Roxihexal
22. Roxithro-lich
23. Roxithromycin
24. Ru 28965
25. Ru 965
26. Ru-28965
27. Ru-965
28. Ru28965
29. Ru965
30. Rulid
1. Roxithromycin
2. 80214-83-1
3. Roxithromycine
4. Roxithromycinum
5. Roxitromicina
6. Ru 965
7. Ru 28965
8. Ru-965
9. Rulid
10. Chebi:48935
11. Nsc-758443
12. Erythromycin 9-(o-((2-methoxyethoxy)methyl)oxime)
13. 21kof230fa
14. Ru-28965
15. 9-(o-((2-methoxyethoxy)methyl)oxime)erythromycin
16. (9e)-erythromycin 9-(o-((2-methoxyethoxy)methyl)oxime)
17. (e)-roxithromycin
18. Roxithromycine [french]
19. Roxithromycinum [latin]
20. Roxitromicina [spanish]
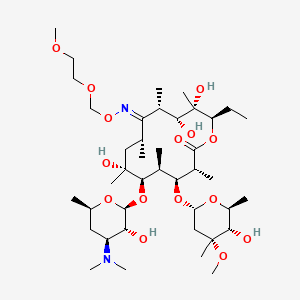
21. (3r,4s,5s,6r,7r,9r,11s,12r,13s,14r,e)-6-(((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-14-ethyl-7,12,13-trihydroxy-4-(((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-10-(((2-methoxyethoxy)methoxy)imino)-3,5,7,9,11,13-hexamethyloxacyclotetradecan-2-one
22. Rulide (tn)
23. 9-[o-[(2-methoxyethoxy)methyl]oxime]erythromycin
24. Sr-05000001850
25. Chebi:48844
26. Unii-21kof230fa
27. Erythromycin, 9-(o-((2-methoxyethoxy)methyl)oxime), (9e)-
28. Ccris 3461
29. Roxithromycin,(s)
30. (3r,4s,5s,6r,7r,9r,10e,11s,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-10
31. Roxithromycin [usan:inn:ban:jan]
32. Rc2952
33. Brn 4286925
34. Spectrum5_001058
35. Roxithromycin [mi]
36. Roxithromycin [inn]
37. Roxithromycin [jan]
38. Roxithromycin [usan]
39. Schembl65985
40. Bspbio_002717
41. Roxithromycin [mart.]
42. Spectrum1503276
43. 9-[o-(2-methoxyethoxymethyl)-oxime] Of Erythromycin
44. Erythromycin, 9-(o-((2-methoxyethoxy)methyl)oxime)
45. Roxithromycin [who-dd]
46. Chembl1214185
47. Hms501d04
48. Hms1922o19
49. Hms2093c11
50. Hms3714l09
51. Pharmakon1600-01503276
52. Roxithromycin (jp17/usan/inn)
53. Roxithromycin [ep Impurity]
54. Hy-b0435
55. Roxithromycin [ep Monograph]
56. Bdbm50248154
57. Ccg-39329
58. Mfcd00214389
59. Nsc758443
60. Zinc96061888
61. Akos015969730
62. Db00778
63. Nsc 758443
64. Idi1_000382
65. Ncgc00178510-01
66. Roxithromycin 100 Microg/ml In Methanol
67. (3r,4s,5s,6r,7r,9r,10e,11s,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-10-(2-methoxyethoxymethoxyimino)-3,5,7,9,11,13-hexamethyl-oxacyclotetradecan-2-one
68. Roxithromycin 1000 Microg/ml In Methanol
69. Sbi-0051809.p002
70. Roxithromycin 100 Microg/ml In Acetonitrile
71. D01710
72. Ab00052342_02
73. Sr-05000001850-1
74. Sr-05000001850-2
75. Brd-k38684403-001-03-9
76. Brd-k38684403-001-05-4
77. Q27895851
78. 9-[o-[(2-methoxyethoxy)methyl]oxime]erythromycin, (9e)-
79. (e)-erythromycin-9-(o-((2-methoxyethoxy)methyl)oxime)
80. Erythromycin 9-(e)-(o-((2-methoxyethoxy)methyl)oxime)
81. Erythromycin A, 9-(o-(2-methoxyethoxymethyl)-oxime)
82. (3r,4s,5s,6r,7r,9r,10e,11s,12r,13s,14r)-4-(2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyloxy)-14-ethyl-7,12,13-trihydroxy-10-{[(2-methoxyethoxy)methoxy]imino}-6-[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyloxy]-3,5,7,9,11,13-hexamethyloxacyclotetradecan-2-one
83. (3r,4s,5s,6r,7r,9r,10e,11s,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyl-tetrahydropyran-2-yl]oxy-10-(2-methoxyethoxymethoxyimino)-3,5,7,9,11,13-hexamethyl-oxacyclotetradecan-2-one
84. (3r,4s,5s,6r,7r,9r,10e,11s,12r,13s,14r)-6-{[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl]oxy}-10-{[(2-methoxyethoxy)m
85. (3r,4s,5s,6r,7r,9r,10e,11s,12r,13s,14r)-6-{[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl]oxy}-10-{[(2-methoxyethoxy)methoxy]imino}-3,5,7,9,11,13-hexamethyloxacyclotetradecan-2-one
86. (3r,4s,5s,6r,7r,9r,10e,11s,12r,13s,14r)-6-{[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl]oxy}-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione 10-{o-[(2-methoxyethoxy)methyl]oxime} (non-preferred Name)
87. (3r,4s,5s,6r,7r,9r,11s,12r,13s,14r)-4-((2,6dideoxy-3-c-methyl-3-o-methyl-.alpha.-l-ribo-hexopyranosyl)oxy)-14-ethyl-7,12,13-trihydroxy-10-((e)((2-methoxyethoxy)methoxy)imino)-3,5,7,9,11,13hexamethyl-6-((3,4,6-trideoxy-3-(dimethylamino)-.beta.-d-xylohexopyranosyl)oxy)oxacyclotetradecan-2-one
88. (3r,4s,5s,6r,7r,9r,11s,12r,13s,14r)-4-(2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyloxy)-14-ethyl-7,12,13-trihydroxy-10-{[(2-methoxyethoxy)methoxy]imino}-6-[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyloxy]-3,5,7,9,11,13-hexamethyloxacyclotetradecan-2-one
89. (3r,4s,5s,6r,7r,9r,11s,12r,13s,14r)-6-{[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy}-3,5,7,9,11,13-hexamethyl-10-(2,4,7-trioxa-1-azaoctan-1-ylidene)-1-oxacyclotetradecan-2-one
90. (3r,4s,5s,6r,7r,9r,11s,12r,13s,14r,e)-6-(((2s,3r,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-14-ethyl-7,12,13-trihydroxy-4-(((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-10-(((2-methoxyethoxy)methoxy)imino)-3,5,7,9,11,13-hexamethyloxacyclotetradecan-2-one
| Molecular Weight | 837.0 g/mol |
|---|---|
| Molecular Formula | C41H76N2O15 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 13 |
| Exact Mass | 836.52456972 g/mol |
| Monoisotopic Mass | 836.52456972 g/mol |
| Topological Polar Surface Area | 217 Ų |
| Heavy Atom Count | 58 |
| Formal Charge | 0 |
| Complexity | 1310 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used to treat respiratory tract, urinary and soft tissue infections.
Roxithromycin has the following antibacterial spectrum in vitro: Streptococcus agalactiae, Streptococcus pneumoniae (Pneumococcus), Neisseria meningitides (Meningococcus), Listeria monocytogenes, Mycoplasma pneumoniae, Chlamydia trachomatis, Ureaplasma urealyticum, Legionella pneumophila, Helicobacter (Campylobacter), Gardnerella vaginalis, Bordetella pertussis, Moraxella catarrhalis (Branhamella Catarrhalis), and Haemophilus ducreyi. Roxithromycin is highly concentrated in polymorphonuclear leukocytes and macrophages, achieving intracellular concentrations greater than those outside the cell. Roxithromycin enhances the adhesive and chemotactic functions of these cells which in the presence of infection produce phagocytosis and bacterial lysis. Roxithromycin also possesses intracellular bactericidal activity.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FA - Macrolides
J01FA06 - Roxithromycin
Absorption
Very rapidly absorbed and diffused into most tissues and phagocytes.
Hepatic. Roxithromycin is only partially metabolised, more than half the parent compound being excreted unchanged. Three metabolites have been identified in urine and faeces: the major metabolite is descladinose roxithromycin, with N-mono and N-di-demethyl roxithromycin as minor metabolites. The respective percentage of roxithromycin and these three metabolites is similar in urine and faeces.
12 hours
Roxithromycin prevents bacterial growth by interfering with their protein synthesis. It binds to the 50S subunit of bacterial ribosomes and inhibits the translocation of peptides.