

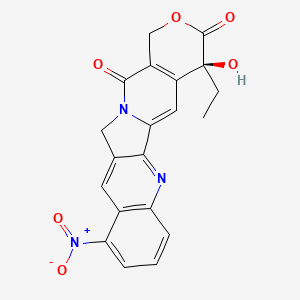

1. 9-nitro-20(s)-camptothecin
2. 9-nitrocamptothecin
3. 9-nitrocamptothecin, (s)-isomer
4. Rfs 2000
5. Rfs-2000
6. Rfs2000
1. 9-nitrocamptothecin
2. 91421-42-0
3. 9-nitro-20(s)-camptothecin
4. Orathecin
5. 9-nc
6. Camptogen
7. Rfs 2000
8. Rfs-2000
9. (s)-4-ethyl-4-hydroxy-10-nitro-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
10. 86639-62-5
11. Nitrocamptothecin
12. Rubitecan [usan]
13. Chebi:90225
14. Rfs2000
15. Rubitecan [usan:inn]
16. Unii-h19c446xxb
17. 9-nitro-(20s)-camptothecin
18. H19c446xxb
19. 9-nitro-20-(s)-camptothecin
20. Dtxsid7046752
21. Rfs 2000;9-nitrocamptothecin
22. (s)-4-ethyl-4-hydroxy-10-nitro-1h-pyrano[3',4':6,7]-indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
23. 9nc
24. Ncgc00167969-01
25. (4s)-4-ethyl-4-hydroxy-10-nitro-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
26. 1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione, 4-ethyl-4-hydroxy-10-nitro-, (4s)-
27. Rubitecanum
28. Inhaled Orathecin
29. C20h15n3o6
30. 1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione, 4-ethyl-4-hydroxy-9-nitro-
31. 1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione, 4-ethyl-4-hydroxy-9-nitro-
32. Rubitecan (inhaled)
33. 9-nitro Camptothecin
34. Rubitecan [inn]
35. Rubitecan [mi]
36. Rubitecan (usan/inn)
37. L9nc
38. D0i5xo
39. D0y1fo
40. Rubitecan [mart.]
41. Rubitecan [who-dd]
42. Schembl8640
43. 9-nitrocamptothecin (9-nc)
44. Ethyl-hydroxy-nitro-[?]dione
45. Chembl77305
46. Rubitecan (inhaled), Supergen
47. Camptothecin, 9-nitro-20(s)
48. Dtxcid5026752
49. Vhxnkpbccmumsw-fqevstjzsa-n
50. Bcp06207
51. Ex-a4326
52. Tox21_112597
53. Bdbm50248354
54. Mfcd06656294
55. S2288
56. Akos015895332
57. Akos025149224
58. C20-h15-n3-o6
59. Db06159
60. St-2617
61. Ncgc00167969-02
62. Ncgc00167969-03
63. Ac-13389
64. Ac-25083
65. Ac-33157
66. As-14856
67. Hy-13744
68. Cas-91421-42-0
69. Cs-0007769
70. N0822
71. D04031
72. A847954
73. Q510113
74. Aerosolized Liposomal 9 Nitro-20 (s) Camptothecin
75. Q-100889
76. Brd-k79821389-001-01-9
77. (19s)-19-ethyl-19-hydroxy-8-nitro-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaene-14,18-dione
78. (s)-4-ethyl-4-hydroxy-10-nitro-1h-pyrano[3\',4\':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
| Molecular Weight | 393.3 g/mol |
|---|---|
| Molecular Formula | C20H15N3O6 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 126 |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 861 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in pancreatic cancer, leukemia (unspecified), melanoma, ovarian cancer, and cancer/tumors (unspecified).
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Topoisomerase I Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE I. (See all compounds classified as Topoisomerase I Inhibitors.)
Rubitecan prevents DNA from unwinding during replication via DNA topoisomerase 1, therefore interfering with tumor growth.