


1. Mk-8408
1. 1613081-64-3
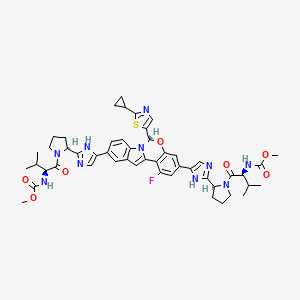
2. Mk-8408
3. Lx752bd95y
4. Carbamic Acid, N,n'-(((6s)-6-(2-cyclopropyl-5-thiazolyl)-1-fluoro-6h-indolo(1,2-c)(1,3)benzoxazine-3,10-diyl)bis(1h-imidazole-5,2-diyl-(2s)-2,1-pyrrolidinediyl((1s)-1-(1-methylethyl)-2-oxo-2,1-ethanediyl)))bis-, C,c'-dimethyl Ester
5. Methyl N-[(2s)-1-[(2s)-2-[5-[(6s)-6-(2-cyclopropyl-1,3-thiazol-5-yl)-1-fluoro-3-[2-[(2s)-1-[(2s)-2-(methoxycarbonylamino)-3-methylbutanoyl]pyrrolidin-2-yl]-1h-imidazol-5-yl]-6h-indolo[1,2-c][1,3]benzoxazin-10-yl]-1h-imidazol-2-yl]pyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate
6. Ruzasvir [usan:inn]
7. Unii-lx752bd95y
8. Ruzasvir [usan]
9. Ruzasvir (usan/inn)
10. Ruzasvir [inn]
11. Ruzasvir [who-dd]
12. Chembl3971095
13. Schembl18268432
14. Db11713
15. J3.601.978f
16. D11217
17. Q27283234
18. Dimethyl ((2s,2's)-((2s,2's)-(((s)-6-(2-cyclopropylthiazol-5-yl)-1-fluoro-6h-benzo[5,6][1,3]oxazino[3,4-a]indole-3,10-diyl)bis(1h-imidazole-5,2-diyl))bis(pyrrolidine-2,1-diyl))bis(3-methyl-1-oxobutane-1,2-diyl))dicarbamate
| Molecular Weight | 947.1 g/mol |
|---|---|
| Molecular Formula | C49H55FN10O7S |
| XLogP3 | 6.4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 14 |
| Exact Mass | 946.39599347 g/mol |
| Monoisotopic Mass | 946.39599347 g/mol |
| Topological Polar Surface Area | 230 Ų |
| Heavy Atom Count | 68 |
| Formal Charge | 0 |
| Complexity | 1840 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)