


1. 2-(dimethylamino)-1-((2-(3-methoxyphenyl)phenoxy)methyl)ethyl Hydrogensuccinate Hydrochloride
2. Mci 9042
3. Mci-9042
4. Mono-(2-(dimethylamino)-1-((2-(2-(3-methoxyphenyl)ethyl)phenoxy)methyl)ethyl)succinate Hydrochloride
1. 125926-17-2
2. Sarpogrelate [inn]
3. Sarpogrelate (inn)
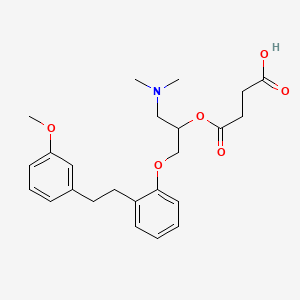
4. 4-[1-(dimethylamino)-3-[2-[2-(3-methoxyphenyl)ethyl]phenoxy]propan-2-yl]oxy-4-oxobutanoic Acid
5. 19p708e787
6. Unii-19p708e787
7. Ncgc00167489-01
8. Ncgc00167489-03
9. Schembl49197
10. Gtpl210
11. Chembl52939
12. Sarpogrelate [who-dd]
13. Dtxsid7048328
14. Chebi:135697
15. Bcp08179
16. Bdbm50093789
17. Stk631325
18. Akos005563803
19. Db12163
20. Ncgc00167489-02
21. 4-{[1-(dimethylamino)-3-{2-[2-(3-methoxyphenyl)ethyl]phenoxy}propan-2-yl]oxy}-4-oxobutanoic Acid
22. Hy-10563
23. Cs-0002654
24. Ft-0712621
25. Ls-187,118
26. D08508
27. Q44931
28. 159s512
29. L000858
30. Sr-01000883998
31. J-513134
32. Sr-01000883998-1
33. (+-)-2-(dimethylamino)-1-((o-(m-methoxyphenethyl)phenoxy)methyl)ethyl Hydrogen Succinate
34. (- )-2-(dimethylamino)-1-((o-(m-methoxyphenethyl)phenoxy)methyl)ethyl Hydrogen Succinate.
35. 4-(1-(dimethylamino)-3-(2-(3-methoxyphenethyl)phenoxy)propan-2-yloxy)-4-oxobutanoic Acid
36. 4-((1-(dimethylamino)-3-(2-(3-methoxyphenethyl)phenoxy)propan-2-yl)oxy)-4-oxobutanoic Acid
37. 4-(1-(2-(3-methoxyphenethyl)phenoxy)-3-(dimethylamino)propan-2-yloxy)-4-oxobutanoic Acid Sarpogrelate
38. 4-[1-dimethylamino-3-[2-[2-(3-methoxyphenyl)ethyl]phenoxy]propan-2-yl]oxy-4-oxobutanoic Acid
39. Butanedioic Acid, Mono(2-(dimethylamino)-1-((2-(2-(3-methoxyphenyl)ethyl)phenoxy)methyl)ethyl) Ester
40. Butanedioic Acid,1-[2-(dimethylamino)-1-[[2-[2-(3-methoxyphenyl)ethyl]phenoxy]methyl]ethyl]ester
41. Succinic Acid Mono-(2-dimethylamino-1-{2-[2-(3-methoxy-phenyl)-ethyl]-phenoxymethyl}-ethyl) Ester; Hydrochloride
| Molecular Weight | 429.5 g/mol |
|---|---|
| Molecular Formula | C24H31NO6 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 14 |
| Exact Mass | 429.21513771 g/mol |
| Monoisotopic Mass | 429.21513771 g/mol |
| Topological Polar Surface Area | 85.3 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 540 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Fibrinolytic Agents
Fibrinolysin or agents that convert plasminogen to FIBRINOLYSIN. (See all compounds classified as Fibrinolytic Agents.)
Serotonin Antagonists
Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. (See all compounds classified as Serotonin Antagonists.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
