

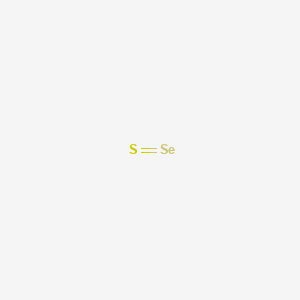
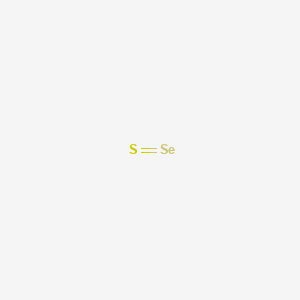
1. Selenium Sulphide
2. 7446-34-6
3. Selenium Sulfide (ses)
4. Selensulfid
5. Selensulfid [german]
6. Ccris 554
7. Hsdb 679
8. Nci-c50033
9. Unii-j90wfr7zff
10. Selenium Sulfide Red Powder
11. Selenosulfide
12. Abbotselsun
13. Caspiselenium
14. Selensulfur
15. Selenenyl Sulfide
16. Selenium Sulfide Usp
17. Ses2
18. Dtxsid9021265
19. 446s346
20. Q27430357
1. Selsun
2. Selenium Disulfide
3. Lenium
| Molecular Weight | 111.04 g/mol |
|---|---|
| Molecular Formula | SSe |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 111.88859 g/mol |
| Monoisotopic Mass | 111.88859 g/mol |
| Topological Polar Surface Area | 32.1 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Selenium sulfide |
| PubMed Health | Selenium Sulfide (On the skin) |
| Drug Classes | Antiseborrheic, Dermatological Agent |
| Drug Label | Each mL contains 23 mg of selenium sulfide in a vehicle consisting of: ammonium lauryl sulfate, caprylic capric triglyceride, chromium oxide green, citric acid, cocamidopropyl betaine, D&C yellow #8, diazolidinyl urea, disodium EDTA, FD&C red #40, |
| Active Ingredient | Selenium sulfide |
| Dosage Form | Lotion/shampoo |
| Route | Topical |
| Strength | 2.5% |
| Market Status | Prescription |
| Company | Wockhardt; Perrigo New York |
| 2 of 4 | |
|---|---|
| Drug Name | Selsun |
| Active Ingredient | Selenium sulfide |
| Dosage Form | Lotion/shampoo |
| Route | Topical |
| Strength | 2.5% |
| Market Status | Prescription |
| Company | Chattem |
| 3 of 4 | |
|---|---|
| Drug Name | Selenium sulfide |
| PubMed Health | Selenium Sulfide (On the skin) |
| Drug Classes | Antiseborrheic, Dermatological Agent |
| Drug Label | Each mL contains 23 mg of selenium sulfide in a vehicle consisting of: ammonium lauryl sulfate, caprylic capric triglyceride, chromium oxide green, citric acid, cocamidopropyl betaine, D&C yellow #8, diazolidinyl urea, disodium EDTA, FD&C red #40, |
| Active Ingredient | Selenium sulfide |
| Dosage Form | Lotion/shampoo |
| Route | Topical |
| Strength | 2.5% |
| Market Status | Prescription |
| Company | Wockhardt; Perrigo New York |
| 4 of 4 | |
|---|---|
| Drug Name | Selsun |
| Active Ingredient | Selenium sulfide |
| Dosage Form | Lotion/shampoo |
| Route | Topical |
| Strength | 2.5% |
| Market Status | Prescription |
| Company | Chattem |
/Experimental Therapy/ ... This randomized, double-blind, placebo-controlled intervention study included 725 institutionalized elderly patients (>65 years) from 25 geriatric centers in France. Patients received an oral daily supplement of nutritional doses of trace elements (zinc and selenium sulfide) or vitamins (beta carotene, ascorbic acid, and vitamin E) or a placebo within a 2 x 2 factorial design for 2 years. ... Correction of specific nutrient deficiencies was observed after 6 months of supplementation and was maintained for the first year, during which there was no effect of any treatment on delayed-type hypersensitivity skin response. Antibody titers after influenza vaccine were higher in groups that received trace elements alone or associated with vitamins, whereas the vitamin group had significantly lower antibody titers (P<.05). The number of patients without respiratory tract infections during the study was higher in groups that received trace elements (P = .06). Supplementation with neither trace elements nor vitamins significantly reduced the incidence of urogenital infections. Survival analysis for the 2 years did not show any differences between the 4 groups. CONCLUSIONS: Low-dose supplementation of zinc and selenium provides significant improvement in elderly patients by increasing the humoral response after vaccination and could have considerable public health importance by reducing morbidity from respiratory tract infections.
PMID:10218756 Girodon F et al; Arch Intern Med 159 (7): 748-54 (1999)
/Experimental Therapy/ Forty children aged 1-11 years with clinically diagnosed tinea capitis were randomized to receive selenium sulfide shampoo 1% or ciclopirox shampoo 1% twice a week as adjuncts to an 8-week course of ultramicronized griseofulvin dosed at 10-12 mg/kg/day. At weeks 2, 4, and 8, subjects returned to the clinic for evaluation and scalp cultures. Subjects then returned for follow-up visits 4 weeks after completing treatment. Overall, by 8 weeks, 30 of 33 (90.9%) treated children demonstrated mycological cure. Selenium sulfide shampoo 1% and ciclopirox shampoo 1% were equally effective as adjunctive treatments for tinea capitis in children in our study.
PMID:20735804 Chen C et al; Pediatr Dermatol 27 (5): 459-62 (2010)
Two cases of new chemicals causing yellow hair shaft discoloration are reported. The chemicals include selenium sulfide 2.5% shampoo and dihydroxyacetone.
PMID:18664164 Prevost N, English JC III; J Drugs Dermatol 7 (77): 689-91 (2008)
... Ingestion is hazardous. If swallowed, avoid oils or alcohol which may promote absorption.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-129
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AE - Other antifungals for topical use
D01AE13 - Selenium sulfide
The percutaneous absorption of selenium sulfide was ... studied in /a/ groups who applied the drug to the entire skin for five minutes for eighteen days. Fluorimetric analysis of urinary samples collected on the third and thirteenth days of treatment revealed no significant increase in the excretion of selenium as compared to pretreatment levels. Systemic toxicity was not observed in any of the patients treated. The results suggest that the selenium sulfide is absorbed poorly from the skin...
PMID:988451 Costa Martins JE et al; Med Cutan Ibero Lat Am 4 (2): 137-41 (1976)

