


1. 563-41-7
2. Aminourea Hydrochloride
3. Hydrazinecarboxamide Hydrochloride
4. Semicarbazide Hcl
5. Hydrazinecarboxamide, Monohydrochloride
6. Semicarbazide Chloride
7. Amidourea Hydrochloride
8. Carbamylhydrazine Hydrochloride
9. Hydrazinecarboxamide Monohydrochloride
10. Semicarbazide Monohydrochloride
11. Carbamyl Hydrazine.hcl
12. Hydrazinecarboxamide, Hydrochloride
13. Ek 226
14. Semicarbazide, Monohydrochloride
15. Aminourea;hydrochloride
16. Semicarbazide, Hydrochloride
17. Semicarbazide (hydrochloride)
18. Chebi:82532
19. Nsc-4732
20. 34854vg84b
21. Ccris 556
22. Hsdb 5197
23. Semicarbazide Hydrochloride (van)
24. Nsc 4732
25. Einecs 209-247-0
26. Semikarbazide Hydrochloride
27. Hydrazinecarboxamide, Hydrochloride (van)
28. Ai3-52680
29. Unii-34854vg84b
30. Hydrazine Carboxamide Hydrochloride
31. Mfcd00013009
32. Semicarbazide Hcl Salt
33. N-aminoureahydrochloride
34. Nh2nhconh2.hcl
35. Semicarbazide Hydrocloride
36. Dsstox_cid_244
37. Semicarbazide-hydrochloride
38. Semicarbazide.hydrochloride
39. Wln: Zvmz &gh
40. Semi-carbazide Hydrochloride
41. Schembl6932
42. Dsstox_rid_75458
43. Dsstox_gsid_20244
44. Mls002153236
45. Hydrochloric Acid Semicarbazide
46. Hydrazinecarboxamidehydrochloride
47. Sca-hcl-13c,15n2
48. Chembl1256355
49. Dtxsid4020244
50. Nsc4732
51. Hydrazinecarbox-amide Hydrochloride
52. Hy-y0470
53. Str00205
54. Semicarbazide Hydrochloride, >=99%
55. Tox21_200950
56. Tox21_501096
57. Akos015848282
58. Ccg-222400
59. Cs-w018530
60. Lp01096
61. Semicarbazide Hydrochloride [mi]
62. Ncgc00091589-01
63. Ncgc00094369-01
64. Ncgc00258503-01
65. Ncgc00261781-01
66. Cas-563-41-7
67. Semicarbazide Hydrochloride [hsdb]
68. Semicarbazide Hydrochloride [iarc]
69. Smr000326716
70. Hydrazinecarboxamide, Hydrochloride (1:1)
71. Semicarbazide Hydrochloride, P.a., 99.0%
72. Eu-0101096
73. Ft-0632395
74. Ft-0674553
75. C19521
76. D77852
77. S 2201
78. S-2460
79. Sr-01000075648
80. Sr-01000075648-2
81. Q27156046
82. F1908-0069
83. Semicarbazide Hydrochloride, Purum P.a., >=98.0% (at)
84. Semicarbazide Hydrochloride, Saj Special Grade, >=99.0%
85. Semicarbazide Hydrochloride, Vetranal(tm), Analytical Standard
86. 873205-62-0
| Molecular Weight | 111.53 g/mol |
|---|---|
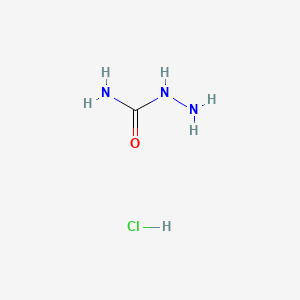
| Molecular Formula | CH6ClN3O |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 111.0199395 g/mol |
| Monoisotopic Mass | 111.0199395 g/mol |
| Topological Polar Surface Area | 81.1 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 42.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
... Binds to cytosine residues in RNA, to deoxycytosine residues in DNA in vitro and to cytosine and deoxycytosine nucleosides
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V12 212
Semicarbazide, a hydrazine derivative, is carcinogenic to mice but shows no or little mutagenicity in the Salmonella-microsome test. To clarify whether or not the genotoxic mechanism contributes to the non-mutagenic carcinogenicity of semicarbazide, ...DNA damage induced by semicarbazide /was investigated/ using 32P-5'-end-labeled DNA fragments obtained from the c-Ha-ras-1 protooncogene and the p53 tumor suppressor gene. Semicarbazide caused DNA damage frequently at the thymine and cytosine residues in the presence of Cu(II). Catalase and bathocuproine partially inhibited DNA damage, suggesting that hydrogen peroxide plus Cu(I) participates in DNA damage. When a high concentration of semicarbazide was used in the presence of catalase, DNA damage was induced, especially at G in 5'-AG and slightly at 5'-G in GG and GGG sequences. An electron paramagnetic resonance (EPR) spectroscopic study has confirmed that the reaction of semicarbazide with Cu(II) produces carbamoyl radicals (.CONH2), possibly generated via the nitrogen-centered radicals of semicarbazide. Azodicarbonamide also produced carbamoyl radicals and induced DNA damage frequently at 5'-G in GG and GGG sequences, suggesting that carbamoyl radicals participate in this sequence-specific DNA damage by semicarbazide. On the basis of... previous reports, ...the sequence-specific DNA damage at G in 5'-AG in the present study is due to the nitrogen-centered radicals. This study has shown that semicarbazide induces DNA damage in the presence of Cu(II) through the formation of hydrogen peroxide and Cu(I). In addition, semicarbazide-derived free radicals participate in DNA damage. DNA damage induced by these reactive species may be relevant to the carcinogenicity of semicarbazide. /Semicarbazide/
Hirakawa K et al; Mut Res/Gen Toxicol and Environ Mutagen 536 (1-2): 91-101 (2003)