
1. Acid, Fusidic
2. Fucithalmic
3. Fusidate, Silver
4. Fusidate, Sodium
5. Fusidic Acid
6. Fusidic Acid, Sodium Salt
7. Fusidin
8. Silver Fusidate
9. Sodium Fusidate
10. Sodium, Fusidate
11. Stanicide
1. Sodium Fusidate
2. 751-94-0
3. Fusidic Acid Sodium Salt
4. Fucidin
5. Fucidine
6. Fusidic Acid, Sodium Salt
7. Fusidic Acid Sodium
8. Fusidate Sodium [usan]
9. Sq 16360
10. Fusidin
11. Fucidin Leo
12. Sq-16360
13. Fusidic Acid (sodium Salt)
14. J7p3696bcq
15. Zn-6
16. Sodium Fusidin
17. Fucidina
18. Fusidate Sodium (usan)
19. Intertulle Fucidin
20. Zn 6-na
21. Mfcd09054714
22. Smr000326707
23. Einecs 212-030-3
24. Oate
25. Unii-j7p3696bcq
26. Sodium Fusitate
27. Fusidicacidsodium
28. Ncgc00017030-01
29. Cas-751-94-0
30. Prestwick_826
31. Fucidin Leo (tn)
32. Sodium Fusidate (jp17)
33. Dsstox_cid_25552
34. Dsstox_rid_80951
35. Dsstox_gsid_45552
36. Schembl74969
37. Mls001076552
38. Mls001333217
39. Mls001333218
40. Mls002153242
41. Mls002207062
42. Sodium 3alpha,11alpha,16beta-trihydroxy-29-nor-8alpha,9beta,13alpha,14beta-dammara-17(20),24-dien-21-oate 16-acetate
43. Sodium Fusidate [jan]
44. Chembl1256987
45. Dtxsid9045552
46. Sodium Fusidate [mart.]
47. Hy-b1350a
48. Fusidate Sodium [who-dd]
49. Hms1569m21
50. Hms2236d08
51. Tox21_110745
52. S4663
53. Akos005146265
54. Fusidic Acid Sodium Salt [mi]
55. Sodium Fusidate [ep Monograph]
56. Ccg-269952
57. Cs-4520
58. Ks-1299
59. 29-nordammara-17(20),24-dien-21-oic Acid, 16-(acetyloxy)-3,11-dihydroxy-, Monosodium Salt, (3.alpha.,4.alpha.,8.alpha.,9.beta.,11.alpha.,13.alpha.,14.beta.,16.beta.,17z)-
60. 29-nordammara-17(20),24-dien-21-oic Acid, 16-(acetyloxy)-3,11-dihydroxy-, Monosodium Salt, (3alpha,4alpha,8alpha,9beta,11alpha,13alpha,14beta,16beta,17z)-
61. C76403
62. D00213
63. A865723
64. Q-201142
65. Q27281314
66. Fusidic Acid Sodium Salt, Antibiotic For Culture Media Use Only
67. Sodium 3.alpha.,11.alpha.,16.beta.-trihydroxy-29-nor-8.alpha.,9.beta.,13.alpha.,14.beta.-dammara-17(20),24-dien-21-oate 16-acetate
68. Sodium;(2z)-2-[(3r,4s,5s,8s,9s,10s,11r,13r,14s,16s)-16-acetyloxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-en
69. Sodium;(2z)-2-[(3r,4s,5s,8s,9s,10s,11r,13r,14s,16s)-16-acetyloxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-enoate
1. Fucidate Sodium
2. Fusidate, Sodium
3. Sodium, Fusidate
4. Fusidic Acid, Sodium Salt
| Molecular Weight | 538.7 g/mol |
|---|---|
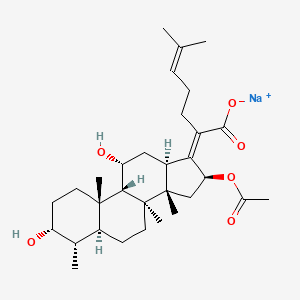
| Molecular Formula | C31H47NaO6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 538.32703350 g/mol |
| Monoisotopic Mass | 538.32703350 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 1000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)