


1. Caustic Soda
2. Hydroxide, Sodium
3. Soda, Caustic
1. Caustic Soda
2. 1310-73-2
3. Sodium Hydrate
4. Aetznatron
5. Soda Lye
6. White Caustic
7. Ascarite
8. Soda, Caustic
9. Natriumhydroxid
10. Rohrputz
11. Sodium Hydroxide Solution
12. Plung
13. Collo-grillrein
14. Liquid-plumr
15. Caustic Soda Solution
16. Collo-tapetta
17. Fuers Rohr
18. Sodium Hydroxide (na(oh))
19. Rohrreiniger Rofix
20. Naoh
21. Hydroxyde De Sodium
22. Natriumhydroxyde
23. Sodium Hydroxide Dimer
24. White Caustic Solution
25. Sodium;hydroxide
26. Sodium Hydrate Solution
27. Sodium Hydroxide, Pellets
28. Sodio(idrossido Di)
29. Sodium(hydroxyde De)
30. Stamperprep
31. Sodium Hydroxide (na2(oh)2)
32. Tosoh Pearl
33. Lye
34. Mfcd00003548
35. Promoter 3308b
36. Sodium Hydroxide, Flake
37. Sodium Hydroxide, Pearl
38. Sodium Hydroxide, Solid
39. Sodium Hydroxide Solutions
40. Nsc-135799
41. Chebi:32145
42. Ins-524
43. 55x04qc32i
44. E-524
45. Soda, Hydrate
46. Natrium Causticum
47. Soda, Kaustische
48. Na (o H)
49. Buffer Solution, Ph 8.00
50. Lewis-red Devil Lye
51. Caustic Soda, Liquid
52. Caswell No. 773
53. Sodiumhydroxide
54. Natriumhydroxid [german]
55. Natriumhydroxyde [dutch]
56. Natrium-hydroxid, Reinstes
57. Un 1823 (solid)
58. Un 1824 (solution)
59. Hydroxyde De Sodium [french]
60. Hsdb 229
61. Sodio(idrossido Di) [italian]
62. Sodium(hydroxyde De) [french]
63. Sodium Hydroxide [nf]
64. Einecs 215-185-5
65. Un1823
66. Un1824
67. Epa Pesticide Chemical Code 075603
68. Nsc 135799
69. Sodiumhydroxid
70. Hydroxyl Sodium
71. Sodium Hydoxide
72. Sodium Hydroxid
73. Sodium Hyroxide
74. Soude Caustique
75. Unii-55x04qc32i
76. Hydroxide Sodium
77. Lye Solution
78. Sodium Hidroxide
79. Sodium Hydroxyde
80. Sodium-hydroxide
81. Natrii Hydroxydum
82. Caustic Soda, Dry
83. Caustic Soda Flake
84. Caustic Soda, Lye
85. Caustic Soda, Bead
86. Caustic Soda, Flake
87. Caustic Soda, Solid
88. Caustic Soda 50%
89. Sodium Hydroxide, Dry
90. 81133-20-2
91. Sodium Hydroxide Beads
92. Caustic Soda, Granular
93. Sodium Hydroxide Liquid
94. Sodium Hydroxide, Bead
95. Sodium Hydroxide 50%
96. Sodium Hydroxide (tn)
97. Sodium Hydroxide (flake)
98. Sodium Hydroxide (naoh)
99. Sodium Hydroxide (liquid)
100. Sodium Hydroxide, Granular
101. Sodium Hydroxide, Solution
102. Wln: Na Q
103. Ec 215-185-5
104. Sodium Hydroxide, Anhydrous
105. 1n Sodium Hydroxide Solution
106. Sodium Hydroxide (granulated)
107. Sodium Hydroxide [ii]
108. Sodium Hydroxide [mi]
109. Sodium Hydroxide (jp17/nf)
110. Sodium Hydroxide [fcc]
111. Sodium Hydroxide [jan]
112. Sodium Hydroxide Solution 25%
113. Sodium Hydroxide [hsdb]
114. Sodium Hydroxide [inci]
115. Chembl2105794
116. Dtxsid0029634
117. Sodium Hydroxide [vandf]
118. Sodium Hydroxide 1n Concentrate
119. Sodium Hydroxide Pellets Usp-nf
120. Sodium Hydroxide [mart.]
121. Sodium Hydroxide 10n Concentrate
122. Sodium Hydroxide [who-dd]
123. Sodium Hydroxide [who-ip]
124. Sodium Hydroxide Solution, 2.5n
125. Bcp26108
126. Nsc135799
127. Sodium Hydroxide 4n Aqueous Solution
128. Sodium Hydroxide Pellets Acs Reagent
129. Akos015913904
130. Akos015951419
131. Sodium Hydroxide Pellets Reagent Grade
132. Db11151
133. Sodium Hydroxide [ep Monograph]
134. Sodium Hydroxide, 10n Aqueous Solution
135. Sodium Hydroxide Solutions [fcc]
136. Natrii Hydroxydum [who-ip Latin]
137. E-33
138. Sodium Hydroxide Pellets Biochemical Grade
139. Sodium Hydroxide, 5% W/v Aqueous Solution
140. Ft-0645105
141. Ft-0689261
142. O0575
143. S0543
144. Sodium Hydroxide Beads Rgt Grade 1kg
145. Sodium Hydroxide, 20% W/v Aqueous Solution
146. Sodium Hydroxide, 25% W/v Aqueous Solution
147. Sodium Hydroxide, 30% W/w Aqueous Solution
148. Sodium Hydroxide, 40% W/v Aqueous Solution
149. Sodium Hydroxide, 50% W/w Aqueous Solution
150. Sodium Hydroxide, 0.1n Standardized Solution
151. Sodium Hydroxide, 0.5n Standardized Solution
152. Sodium Hydroxide, 1.0n Standardized Solution
153. Sodium Hydroxide, 2.0n Standardized Solution
154. Sodium Hydroxide, 5.0n Standardized Solution
155. Sodium Hydroxide, Roti?volum, 1n (ampoule)
156. D01169
157. Sodium Hydroxide, 0.01n Standardized Solution
158. Sodium Hydroxide, 0.05n Standardized Solution
159. Sodium Hydroxide, 10.0n Standardized Solution
160. Sodium Hydroxide, Roti?volum, 0.1n (ampoule)
161. Sodium Hydroxide, Solid [un1823] [corrosive]
162. Q102769
163. J-005935
164. Sodium Hydroxide, Solution [un1824] [corrosive]
165. Sodium Hydroxide, Pellets, Trace Metals Grade 99.99%
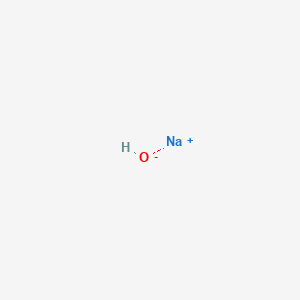
| Molecular Weight | 39.997 g/mol |
|---|---|
| Molecular Formula | HNaO |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 39.99250893 g/mol |
| Monoisotopic Mass | 39.99250893 g/mol |
| Topological Polar Surface Area | 1 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Caustics
National Library of Medicine's Medical Subject Headings online file (MeSH, 2011)
Vet: Caustic, dehorning of calves.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1485
VET: A 2% solution of soda lye (contains 94% sodium hydroxide) in hot water is used as a disinfectant against many common pathogens, such as those causing fowl cholera and pullorum disease.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2153
Used to destroy or kill the nail matrix (matrixectomies).
Sodium Hydroxide 10% forms a strongly alkaline and caustic solution. As a caustic agent, it is used to destroy organic tissue by chemical action.
Caustics
Strong alkaline chemicals that destroy soft body tissues resulting in a deep, penetrating type of burn, in contrast to corrosives, that result in a more superficial type of damage via chemical means or inflammation. Caustics are usually hydroxides of light metals. SODIUM HYDROXIDE and potassium hydroxide are the most widely used caustic agents in industry. Medically, they have been used externally to remove diseased or dead tissues and destroy warts and small tumors. The accidental ingestion of products (household and industrial) containing caustic ingredients results in thousands of injuries per year. (See all compounds classified as Caustics.)
Absorption
There are no quantitative data for the absorption of sodium hydroxide through the skin. Solutions which contain 50 % sodium hydroxide have been shown to be corrosive and lethal when applied dermally to mice.
ALKALIS PENETRATE SKIN SLOWLY.
Dreisbach, R. H. Handbook of Poisoning. 9th ed. Los Altos, California: Lange Medical Publications, 1977., p. 202
Ammonium hydroxide penetrates fastest, followed by sodium hydroxide, potassium hydroxide, and finally calcium hydroxide.
Sullivan, J.B. Jr., G.R. Krieger (eds.). Hazardous Materials Toxicology-Clinical Principles of Environmental Health. Baltimore, MD: Williams and Wilkins, 1992., p. 433
Because of its high-level alkalinity, sodium hydroxide in aqueous solution directly causes bond breakage in proteins (especially disulfide bridges). Hair and fingernails are found to be dissolved after 20 hours of direct contact with sodium hydroxide at pH values higher than 9.2. Sodium hydroxide has depilatory effects which have been described after accidental contact with solutions in the workplace. The breakage of bonds in proteins may lead to severe necrosis to the application site. The level of corrosion depends on the period of contact with the tissue, and on the concentration of sodium hydroxide.