


1. Chenyl Taurine Sodium
2. Tauroursodeoxycholate
3. Tauroursodeoxycholate Sodium Salt
4. Tauroursodeoxycholic Acid
5. Tauroursodeoxycholic Acid, (3alpha,5alpha,7alpha)-isomer
6. Tauroursodeoxycholic Acid, Monosodium Salt, (3alpha,5beta,7alpha)-isomer
7. Taurursodiol
8. Tudca
9. Ursodoxicoltaurine
1. 35807-85-3
2. Ursodeoxycholyltaurine Sodium
3. Tauroursodeoxycholic Acid Sodium Salt
4. Sodium Tauroursodeoxycholate (tudc)
5. Tauroursodeoxycholate Sodium
6. Sodium Tauroursodesoxycholate
7. Tauroursodeoxycholate (sodium)
8. Tauroursodeoxycholate Sodium Salt
9. 6x4jlr867n
10. Sodium Taurochenodesoxycholate
11. Taurochenodeoxycholic Acid Sodium Salt
12. Tudca Sodium
13. Tauroursodeoxycholic Acid Sodium Salt (90%)
14. P2sd3phq3y
15. Chenyl Taurine Sodium
16. Unii-6x4jlr867n
17. Tudc
18. Nsc 681055
19. Unii-p2sd3phq3y
20. Tauroursodeoxycholatesodium
21. Taurursodiol Sodium
22. Ursodoxicoltaurine Sodium
23. Schembl10695686
24. Tauroursodeoxycholic Acidsodium Salt
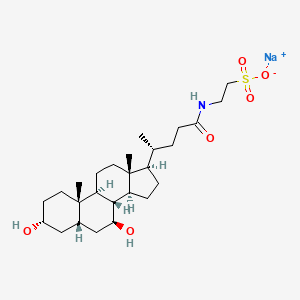
25. Sodium;2-[[(4r)-4-[(3r,5s,7s,8r,9s,10s,13r,14s,17r)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]ethanesulfonate
26. Hy-19696a
27. S7896
28. Akos016005356
29. Ccg-269868
30. Cs-5351
31. 2-(((3alpha,5beta,7alpha)-3,7-dihydroxy-24-oxocholan-24-yl)amino)ethanesulfonic Acid Monosodium Salt
32. Ethanesulfonic Acid, 2-(((3alpha,5beta,7alpha)-3,7-dihydroxy-24-oxocholan-24-yl)amino)-, Monosodium Salt
33. Ethanesulfonic Acid, 2-(((3alpha,5beta,7beta)-3,7-dihydroxy-24-oxocholan-24-yl)amino)-, Sodium Salt (1:1)
34. Ursodoxicoltaurine Sodium [who-dd]
35. C74842
36. Q27265654
37. 3a,7ss-dihydroxy-5ss-cholan-24-oic Acid N-(2-sulfoethyl)amide
38. Ethanesulfonic Acid, 2-(((3.alpha.,5.beta.,7.beta.)-3,7-dihydroxy-24-oxocholan-24-yl)amino)-, Monosodium Salt
39. Ethanesulfonic Acid, 2-(((3.alpha.,5.beta.,7.beta.)-3,7-dihydroxy-24-oxocholan-24-yl)amino)-, Sodium Salt (1:1)
| Molecular Weight | 521.7 g/mol |
|---|---|
| Molecular Formula | C26H44NNaO6S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 521.27870358 g/mol |
| Monoisotopic Mass | 521.27870358 g/mol |
| Topological Polar Surface Area | 135 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 864 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Cholagogues and Choleretics
Gastrointestinal agents that stimulate the flow of bile into the duodenum (cholagogues) or stimulate the production of bile by the liver (choleretic). (See all compounds classified as Cholagogues and Choleretics.)