


1. Antimony Gluconate Sodium
2. Antimony Gluconic Acid
3. Antimony Sodium Gluconate
4. Antimony Sodium Gluconates
5. Myostibin
6. Pentostam
7. Sodium Gluconates, Antimony
8. Sodium, Stibogluconate
9. Solustibosan
10. Stibatin
11. Stibogluconate Sodium
12. Stibogluconate, Sodium
13. Triostam
1. Antimony Sodium Gluconate
2. Solusurmin
3. Stibogluconate Sodium
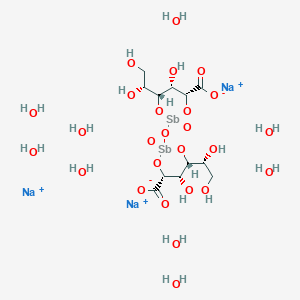
4. Trisodium;(4r,5s,6r)-2-[[(4r,5s,6r)-4-carboxylato-6-[(1r)-1,2-dihydroxyethyl]-5-hydroxy-2-oxo-1,3,2lambda5-dioxastibinan-2-yl]oxy]-6-[(1r)-1,2-dihydroxyethyl]-5-hydroxy-2-oxo-1,3,2lambda5-dioxastibinane-4-carboxylate;nonahydrate
5. Solyusurmin
6. Solustin
7. Stibogluconate Sodique
8. Estibogluconato Sodico [spanish]
9. Natrii Stibogluconas [inn-latin]
10. Estibogluconato Sodico [inn-spanish]
11. Sodium Stibogluconate [inn:ban:dcf]
12. Stibogluconate De Sodium [inn-french]
13. Antimony(v) Derivative Of Sodium Gluconate
14. Unii-apj6285y89
15. Trinatrium Bis(gluconato(3)-o2,o3,o4)hydroxooxido-oxy-bis-antimonat(v)
16. D-gluconic Acid, 2,4:2',4'-o-(oxydistibylidyne)bis-, Sb,sb'-dioxide, Trisodium Salt, Nonahydrate
| Molecular Weight | 908.89 g/mol |
|---|---|
| Molecular Formula | C12H36Na3O26Sb2+ |
| Hydrogen Bond Donor Count | 15 |
| Hydrogen Bond Acceptor Count | 26 |
| Rotatable Bond Count | 6 |
| Exact Mass | 908.92682 g/mol |
| Monoisotopic Mass | 906.92641 g/mol |
| Topological Polar Surface Area | 291 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 1 |
| Complexity | 699 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 13 |
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
Schistosomicides
Agents that act systemically to kill adult schistosomes. (See all compounds classified as Schistosomicides.)
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01C - Agents against leishmaniasis and trypanosomiasis
P01CB - Antimony compounds
P01CB02 - Sodium stibogluconate