
1. Tak-981
2. 1858276-04-6
3. Subasumstat [usan]
4. Xq43h3v6m1
5. Sumoylation Inhibitor Tak-981
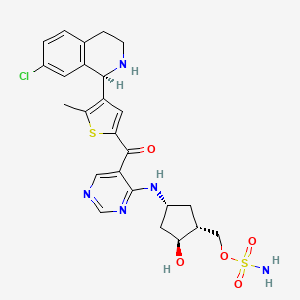
6. [(1r,2s,4r)-4-[[5-[[4-((1r)-7-chloro-1,2,3,4-tetrahydroisoquinolin-1-yl)-5-methylthien-2-yl]carbonyl]pyrimidin-4-yl]amino]-2-hydroxycyclopentyl]methyl Sulfamate
7. 1858276-04-6 (free)
8. ((1r,2s,4r)-4-((5-(4-((r)-7-chloro-1,2,3,4-tetrahydroisoquinolin-1-yl)-5-methylthiophene-2-carbonyl)pyrimidin-4-yl)amino)-2-hydroxycyclopentyl)methyl Sulfamate
9. ((1r,2s,4r)-4-((5-((4-((1r)-7-chloro-1,2,3,4-tetrahydroisoquinolin-1-yl)-5-methylthien-2-yl)carbonyl)pyrimidin-4-yl)amino)-2-hydroxycyclopentyl)methyl Sulfamate
10. [(1r,2s,4r)-4-[(5-[4-[(1r)-7-chloro-1,2,3,4-tetrahydroisoquinolin-1-yl]-5-methylthiophene-2-carbonyl]pyrimidin-4-yl)amino]-2-hydroxycyclopentyl]methyl Sulfamate
11. [(1r,2s,4r)-4-[[5-[4-[(1r)-7-chloro-1,2,3,4-tetrahydroisoquinolin-1-yl]-5-methylthiophene-2-carbonyl]pyrimidin-4-yl]amino]-2-hydroxycyclopentyl]methyl Sulfamate
12. Tak981 Oxalate
13. Tak-981 Oxalate
14. Subasumstat [inn]
15. Subasumstat (proposed Inn)
16. Unii-xq43h3v6m1
17. Subasumstat [who-dd]
18. Tak981
19. Chembl4862901
20. Schembl17398333
21. Gtpl11305
22. Bdbm462958
23. Tak 981; Tak981
24. Bcp30806
25. Ex-a3291
26. Mfcd32062692
27. Nsc820784
28. Who 11858
29. Nsc-820784
30. Us10780090, Compound I-263a
31. Us10780090, Compound I-263b
32. Ac-35773
33. Example 133 [wo2016004136a1]
34. Hy-111789
35. Cs-0091878
36. D81004
37. A935089
38. I-263a [wo2016004136a1]
39. [(1r,2s,4r)-4-[[5-[4-[(1r)-7-chloro-1,2,3,4-tetrahydroisoquinolin-1-yl]-5-methyl-thiophene-2-carbonyl]pyrimidin-4-yl]amino]-2-hydroxy-cyclopentyl]methyl Sulfamate
40. [(1r,2s,4r)-4-{[5-({4-[(1r)-7-chloro-1,2,3,4-tetrahydroisoquinolin-1-yl]-5-methyl-2- Thienyl}carbonyl)pyrimidin-4-yl]amino}-2-hydroxycyclopentyl]methyl Sulfamate
41. Sulfamic Acid, ((1r,2s,4r)-4-((5-((4-((1r)-7-chloro-1,2,3,4-tetrahydro-1-isoquinolinyl)-5-methyl-2-thienyl)carbonyl)-4-pyrimidinyl)amino)-2-hydroxycyclopentyl)methyl Ester
| Molecular Weight | 578.1 g/mol |
|---|---|
| Molecular Formula | C25H28ClN5O5S2 |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Exact Mass | 577.1220391 g/mol |
| Monoisotopic Mass | 577.1220391 g/mol |
| Topological Polar Surface Area | 193 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 942 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Subasumstat binds and forms an adduct with SUMO, stopping the protein from transferring from the SUMO-activating enzyme to SUMO-conjugating enzyme UBC9. Downstream, this stops many sumoylated protein-mediated pathways from occurring in tumor cells, like DNA repair, metastasis, and proliferation. Subasumstat is also able to increase the production of type 1 interferon, which activates antitumor immune responses in cells and signals for increased tumor cell death.