

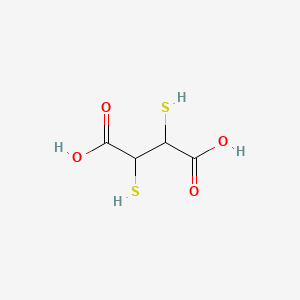

1. 2,3 Dimercaptosuccinic Acid
2. Acid, 2,3-dimercaptosuccinic
3. Acid, Dimercaptosuccinic
4. Acid, Meso-dimercaptosuccinic
5. Butanedioic Acid, 2,3-dimercapto-, (r*,s*)-isomer
6. Chemet
7. Dimercaptosuccinate, Tin
8. Dimercaptosuccinic Acid
9. Dipotassium Salt Succimer
10. Disodium Salt Succimer
11. Dmsa
12. Meso Dimercaptosuccinic Acid
13. Meso-dimercaptosuccinic Acid
14. Monosodium Salt Succimer
15. Rhenium Salt Succimer
16. Ro 1 7977
17. Ro-1-7977
18. Ro17977
19. Succicaptal
20. Succimer
21. Succimer Antimony Sodium Salt, (r*,s*)-isomer
22. Succimer, (r*,r*)-(+,-)-isomer
23. Succimer, Dipotassium Salt
24. Succimer, Disodium Salt
25. Succimer, Monosodium Salt
26. Succimer, Rhenium Salt
27. Succimer, Tin Salt
28. Tin Dimercaptosuccinate
29. Tin Salt Succimer
1. 2418-14-6
2. Dimercaptosuccinic Acid
3. 2,3-bis(sulfanyl)butanedioic Acid
4. Suximer
5. 2,3-dimercaptobutanedioic Acid
6. Butanedioic Acid, 2,3-dimercapto-
7. Dimercaptosuccinicacid
8. Dmsa
9. 2,2-dimercaptosuccinic Acid
10. Dimercaptosuccinic Acid;dmsa
11. Mls001076671
12. Smr000499575
13. Dimercatposuccinic Acid
14. Nsc16866
15. Succinic Acid, 2,3-dimercapto-
16. 3-03-00-01033 (beilstein Handbook Reference)
17. Einecs 219-334-5
18. Nsc 259951
19. Brn 1725149
20. Unii-4s9ju7xf01
21. 2,3-dimercapto-succinic Acid
22. Imercaptosuccinic Acid
23. Cid_9354
24. 2,3-disulfanylsuccinic Acid
25. Succinic Acid,3-dimercapto-
26. Schembl14942
27. Mls001332563
28. Mls001332564
29. Chembl28721
30. Meso-2,3-dithiosuccinic Acid
31. 2,3-disulfanylbutanedioic Acid
32. Butanedioic Acid,3-dimercapto-
33. 2,3-disulfanylsuccinic Acid #
34. 4s9ju7xf01
35. Bdbm60868
36. Dtxsid10859324
37. Meso 2,3-dimercaptosuccinic Acid
38. Nsc259951
39. Mpi Dmsa Kidney Reagent (salt/mix)
40. Akos006228463
41. .alpha.,.beta.-dimercaptosuccinic Acid
42. Db14089
43. Nsc-259951
44. Ls-12987
45. Nci60_001347
46. A4995
47. Ft-0628241
48. Ft-0695306
49. 18d146
50. Q56604713
| Molecular Weight | 182.2 g/mol |
|---|---|
| Molecular Formula | C4H6O4S2 |
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 181.97075102 g/mol |
| Monoisotopic Mass | 181.97075102 g/mol |
| Topological Polar Surface Area | 76.6 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 139 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Mpi dmsa kidney reagent |
| Active Ingredient | Technetium tc-99m succimer kit |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | n/a |
| Market Status | Prescription |
| Company | Ge Healthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Mpi dmsa kidney reagent |
| Active Ingredient | Technetium tc-99m succimer kit |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | n/a |
| Market Status | Prescription |
| Company | Ge Healthcare |
Antidotes
Agents counteracting or neutralizing the action of POISONS. (See all compounds classified as Antidotes.)
Chelating Agents
Chemicals that bind to and remove ions from solutions. Many chelating agents function through the formation of COORDINATION COMPLEXES with METALS. (See all compounds classified as Chelating Agents.)
