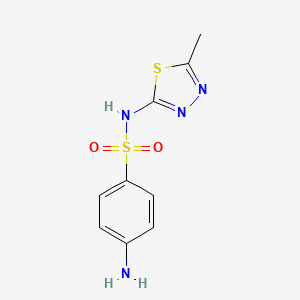

1. Fna, Sulfamethizol
2. Rufol
3. Sulfamethizol Fna
4. Sulphamethizole
5. Thiosulfil
1. 144-82-1
2. Sulfamethizol
3. Sulphamethizole
4. Thiosulfil
5. Rufol
6. Sulfamethylthiadiazole
7. Proklar
8. 4-amino-n-(5-methyl-1,3,4-thiadiazol-2-yl)benzenesulfonamide
9. Sulfamethizolum
10. Sulfametizol
11. Benzenesulfonamide, 4-amino-n-(5-methyl-1,3,4-thiadiazol-2-yl)-
12. 4-amino-n-(5-methyl-1,3,4-thiadiazol-2-yl)benzene-1-sulfonamide
13. Chebi:9331
14. Sulfamethizole (proklar)
15. Urodiaton
16. Uroz
17. Nsc-757327
18. Sulfa Gram
19. Chembl1191
20. Mls000028603
21. Solfametizolo
22. Solfametizolo [dcit]
23. Ncgc00016407-05
24. Ncgc00016407-08
25. Cas-144-82-1
26. Smr000058666
27. Sulfametizol [inn-spanish]
28. Sulfamethizolum [inn-latin]
29. 25w8454h16
30. Dsstox_cid_3615
31. Dsstox_rid_77111
32. Dsstox_gsid_23615
33. Thiosulfil-a-forte
34. Thiosulfil (tn)
35. Ccris 756
36. Hsdb 4379
37. 2-methyl-5-sulfanilamido-1,3,4-thiadiazole
38. Sr-01000003158
39. Einecs 205-641-1
40. N1-(5-methyl-1,3,4-thiadiazol-2-yl)sulfanilamide
41. Sulphamethazole
42. Sulphamethiozole
43. Ai3-50149
44. N(sup 1)-(5-methyl-1,3,4-thiadiazol-2-yl)-sulfanilamide
45. Unii-25w8454h16
46. Prestwick_114
47. Sulfamethizole [usp:inn:ban:jan]
48. N(sup 1)-(5-methyl-1,3,4-thiadiazol-2-yl)sulfanilamide
49. Spectrum_000992
50. Opera_id_1595
51. Prestwick0_000742
52. Prestwick1_000742
53. Prestwick2_000742
54. Prestwick3_000742
55. Spectrum2_001322
56. Spectrum3_000570
57. Spectrum4_000640
58. Spectrum5_001078
59. Epitope Id:122233
60. Sulfamethizole [mi]
61. Sulfamethizole [inn]
62. Sulfamethizole [jan]
63. Schembl26453
64. Bspbio_000724
65. Bspbio_001960
66. Kbiogr_001260
67. Kbioss_001472
68. Sulfamethizole [hsdb]
69. Mls002303066
70. Divk1c_000141
71. Spectrum1500549
72. Sulfamethizole [vandf]
73. Spbio_001443
74. Spbio_002663
75. Sulfamethizole [mart.]
76. Sulfamethizole-(phenyl-13c6)
77. Bpbio1_000798
78. N(1)-(5-methyl-1,3,4-thiadiazol-2-yl)sulfanilamide
79. Sulfamethizole [usp-rs]
80. Sulfamethizole [who-dd]
81. Dtxsid5023615
82. Hms500h03
83. Kbio1_000141
84. Kbio2_001472
85. Kbio2_004040
86. Kbio2_006608
87. Kbio3_001460
88. Zinc57493
89. Ninds_000141
90. Sulfamethizole (jp17/usp/inn)
91. Hms1570e06
92. Hms1921a19
93. Hms2092i21
94. Hms2097e06
95. Hms2233l09
96. Hms3371a13
97. Hms3655f05
98. Hms3714e06
99. Pharmakon1600-01500549
100. Sulfanilamide, N(sup 1)-(5-methyl-1,3,4-thiadiazol-2-yl)-
101. Hy-b0333
102. Sulfamethizole [ep Impurity]
103. Sulfamethizole [orange Book]
104. Tox21_110425
105. Tox21_201035
106. Bdbm50295558
107. Ccg-39260
108. Mfcd00053363
109. Nsc757327
110. S1957
111. Sulfamethizole [ep Monograph]
112. Sulfamethizole [usp Monograph]
113. Akos002666345
114. Tox21_110425_1
115. Db00576
116. Nsc 757327
117. Idi1_000141
118. Smp1_000284
119. Ncgc00016407-01
120. Ncgc00016407-02
121. Ncgc00016407-03
122. Ncgc00016407-04
123. Ncgc00016407-06
124. Ncgc00016407-07
125. Ncgc00016407-09
126. Ncgc00016407-11
127. Ncgc00016407-13
128. Ncgc00024107-03
129. Ncgc00024107-04
130. Ncgc00024107-05
131. Ncgc00258588-01
132. Sulfamethizol 100 Microg/ml In Methanol
133. As-10177
134. Sbi-0051523.p003
135. Ab00052098
136. B2039
137. Sw196425-3
138. 4-(aminomethyl)-1-n-boc-piperidine-hcl
139. C08050
140. D00870
141. D88000
142. Ab00052098_15
143. Ab00052098_16
144. A921093
145. Sulfamethizole, Vetranal(tm), Analytical Standard
146. J-008011
147. Q3976824
148. Sr-01000003158-2
149. Sr-01000003158-3
150. Sulfamethizole, Analytical Standard, >=99% (hplc)
151. Brd-k31682896-001-05-9
152. Brd-k31682896-001-15-8
153. Sulfamethizole, European Pharmacopoeia (ep) Reference Standard
154. 4-amino-n-(5-methyl[1,3,4]thiadiazol-2-yl)-benzenesulfonamide
155. 4-amino-n-[5-methyl-1,3,4-thiadiazol-2-yl]-benzenesulfonamide
156. Benzenesulfonamide,4-amino-n-(5-methyl-1,3,4-thiadiazol-2-yl)-
157. N (sup 1)-(5-methyl-1,3,4-thiadiazol-2-yl)sulfanilamide
158. Sulfamethizole, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 270.3 g/mol |
|---|---|
| Molecular Formula | C9H10N4O2S2 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 270.02451792 g/mol |
| Monoisotopic Mass | 270.02451792 g/mol |
| Topological Polar Surface Area | 135 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 349 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents /SRP: Antibacterial/
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Sulfamethizole is indicated in the treatment of urinary tract infections (primarily pyelonephritis, pyelitis, and cystitis) in the absence of obstructive uropathy or foreign bodies, when these infections are caused by susceptible strains of the following organisms: Escherichia coli, Klebsiella-Enterobacter, Staphylococcus aureus, Proteus mirabilis, and Proteus vulgaris. /Included in US product label/
Medical Economics Co; Physicians Desk Reference: Generics 2nd ed p.2825 (1996)
Sulfonamides are indicated in the treatment of chancroid caused by Hemophilus ducreyi. However, other agents such as erythromycin and ceftriaxone, are considered to be first line agents. /Sulfonamides; Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2660
Sulfonamides are indicated in the treatment of endocervical and urethral infections caused by Chlamydia trachomatis. However, other agents, such as doxycycline and azithromycin, are considered to be first line agents. /Sulfonamides; Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2660
For more Therapeutic Uses (Complete) data for SULFAMETHIZOLE (17 total), please visit the HSDB record page.
The number of conditions for which the sulfonamides are therapeutically useful and constitute drugs of first choice has been reduced sharply by the development of more effective antimicrobial agents and by the gradual increase in the resistance of a number of bacterial species to this class of drugs. /Sulfonamides/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1062
Many of the adverse effects that have been attributed to the sulfonamides appear to be hypersensitivity reactions. The incidence of hypersensitivity reactions appears to increase with increased sulfonamide dosage. Although cross-sensitization has been reported to occur between the various anti-infective sulfonamides, some diuretics such as acetazolamide and the thiazides, some goitrogens, and sulfonylurea antidiabetic agents, the association between hypersensitivity to sulfonamide anti-infectives and subsequent sensitivity reactions to non-anti-infective sulfonamides (e.g., thiazides, sulfonylurea antidiabetic agents, furosemide, dapsone, probenecid) appears to result from a predisposition to allergic reactions in general rather than to cross-sensitivity to the sulfa moiety per se. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 423
Various dermatologic reactions, including rash, pruritus, urticaria, erythema nodosum, erythema multiforme (Stevens-Johnson syndrome), Lyell's syndrome (may be associated with corneal damage), Behcet's syndrome, toxic epidermal necrolysis, and exfoliative dermatitis, have been reported in patients receiving sulfonamides. Because photosensitivity may also occur, patients should be cautioned against exposure to UV light or prolonged exposure to sunlight. A relatively high proportion of fatalities has occurred as a result of the Stevens-Johnson syndrome, especially in children. Although long-acting sulfonamides (which are no longer commercially available) have been associated most often with the Stevens-Johnson syndrome, other sulfonamides also have been reported to cause this reaction. The physician should be alert to the signs, including high fever, severe headache, stomatitis, conjunctivitis, rhinitis, urethritis, and balanitis, which may precede the onset of the cutaneous lesions of the Stevens-Johnson syndrome. If a rash develops during therapy, the sulfonamide should be discontinued at once. In rare instances, a skin rash may precede a more serious reaction such as Stevens-Johnson syndrome, toxic epidermal necrolysis, hepatic necrosis, and/or serious blood disorders. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 423
Fever, which may develop 7-10 days after the initial sulfonamide dose, is a common adverse effect of sulfonamide therapy. Serum sickness syndrome or serum sickness-like reactions (e.g., fever, chills, rigors, flushing, joint pain, urticarial eruptions, conjunctivitis, bronchospasm, leukopenia), have been reported; rarely, anaphylactoid reactions and anaphylaxis may occur. Lupus erythematosus-like syndrome, disseminated lupus erythematosus, angioedema, vasculitis, vascular lesions including periarteritis nodosa and arteritis, cough, shortness of breath, chills, pulmonary infiltrates, pneumonitis (which may be associated with eosinophilia), fibrosing alveolitis, pleuritis, pericarditis with or without tamponade, allergic myocarditis, hepatitis, hepatic necrosis with or without immune complexes, parapsoriasis varioliformis acuta, alopecia, conjunctival and scleral injection, periorbital edema, and arthralgia have also been reported. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 423
For more Drug Warnings (Complete) data for SULFAMETHIZOLE (27 total), please visit the HSDB record page.
For the treatment of urinary tract infection
Sulfamethizole is a sulfonamide antibiotic. The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
B - Blood and blood forming organs
B05 - Blood substitutes and perfusion solutions
B05C - Irrigating solutions
B05CA - Antiinfectives
B05CA04 - Sulfamethizole
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BA - Sulfonamides
D06BA04 - Sulfamethizole
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01EB - Short-acting sulfonamides
J01EB02 - Sulfamethizole
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AB - Sulfonamides
S01AB01 - Sulfamethizole
Absorption
Rapidly absorbed.
Sulfamethizole is readily absorbed form the GI tract. Following oral administration of a single 2-g dose of sulfamethizole in a limited number of patients, blood concentrations of approximately 30 ug/mL were attained within 1 hour. Peak blood concentrations of about 60 ug/mL were reached within 2 hours followed by a gradual decrease to 6.6 ug/mL within eight hours and to 5 ug/mL within 12 hours. Approximately 2-11% of sulfamethizole present in the blood is in the N4-acetylated form.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 621
Sulfamethizole is distributed into most body tissues but does not appear to diffuse into the CSF of patients with normal meninges. Sulfamethizole is approximately 90% bound to plasma proteins. Since the drug is rapidly eliminated in urine, the manufacturer states that accumulation of sulfamethizole in tissues outside the urinary tract is minimal
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 621
Sulfamethizole...is a rapidly eliminated sulfonamide; concentrations of drug in blood are thus low after administration of conventional doses.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 1111
Approximately 80% of an administered dose is recoverable within eight hours; approximately 98% is cleared in 15 to 24 hours. Sulfamethizole is cleared by the kidney at a rate only 10 to 20% lower than that of creatinine.
Medical Economics Co; Physicians Desk Reference: Generics 2nd ed p.2825 (1996)
For more Absorption, Distribution and Excretion (Complete) data for SULFAMETHIZOLE (21 total), please visit the HSDB record page.
Hepatic.
Approximately 95% of a given dose of sulfamethizole is not metabolized; less than 5% is acetylated. As a consequence, almost all of a given dose of sulfamethizole is present in its active form in the body.
Medical Economics Co; Physicians Desk Reference: Generics 2nd ed p.2825 (1996)
Renal clearance values of metabolite N4-acetylsulfonamides are 6-20 times higher than their parent compd. Sulfamethizole is acetylated very little.
PMID:7389236 VREE TB ET AL; CLIN PHARMACOKINET 5 (3): 274 (1980)
Although the liver is the major site of metabolism, sulfonamides may also be metabolized in other body tissues. Most sulfonamides are metabolized mainly by N4-acetylation. The degree of acetylation, which is a function of time, varies from less than 5% for sulfamethizole to up to 40% for sulfadiazine. The N4-acetyl metabolites, which do not possess antibacterial activity, have greater affinity for plasma albumin than does the nonacetylated drug and are usually less soluble than the parent sulfonamide, particularly in acidic urine. Like acetyl derivatives, glucuronide derivatives do not possess antibacterial activity; however, glucuronide derivatives are water soluble, appear to resemble the nonacetylated sulfonamide in plasma binding capacity, and have not been associated with adverse effects. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
3-8 hours
Sulfonamides are generally classified as short-acting, intermediate-acting, or long-acting depending on the rate at which they are absorbed and eliminated. Sulfamethizole, sulfasalazine, and sulfisoxazole are generally considered to be short-acting sulfonamides and reportedly have plasma half-lives of about 4-8 hours. Sulfadiazine and sulfapyridine are generally considered to be intermediate-acting sulfonamides and reportedly have plasma half-lives of about 7-17 hours. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
Sulfamethizole is a competitive inhibitor of bacterial enzyme dihydropteroate synthetase. The normal para-aminobenzoic acid (PABA) substrate is prevented from binding. The inhibited reaction is necessary in these organisms for the synthesis of folic acid.
Sulfonamides are usually bacteriostatic in action. Sulfonamides interfere with the utilization of p-aminobenzoic acid (PABA) in the biosynthesis of tetrahydrofolic acid (the reduced form of folic acid) cofactors in susceptible bacteria. Sulfonamides are structural analogs of PABA and appear to interfere with PABA utilization by competitively inhibiting the enzyme dihydropteroate synthase, which catalyzes the formation of dihydropteroic acid (a precursor of tetrahydrofolic acid) from PABA and pteridine; however, other mechanism(s) affecting the biosynthetic pathway also may be involved. Compounds such as pyrimethamine and trimethoprim, which block later stages in the synthesis of folic acid, act synergistically with sulfonamides. Only microorganisms that synthesize their own folic acid are inhibited by sulfonamides; animal cells and bacteria which are capable of utilizing folic acid precursors or preformed folic acid are not affected by these drugs. The antibacterial activity of the sulfonamides is reportedly decreased in the presence of blood or purulent body exudates. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 424
The sulfonamides are structural analogs of para-aminobenzoic acid (PABA) and competitively inhibit an enzymatic step (dihydropterate synthetase) during which PABA is incorporated into the synthesis of dihydrofolic acid (folic acid). Because dihydrofolate synthesis is reduced, the levels of tetrahydrofolate (folinic acid) formed from dihydrofolate diminish. Tetrahydrofolate is an essential component of the coenzymes responsible for single carbon metabolism in cells. Acting as antimetabolites to PABA, sulfonamides eventually block, in a complex fashion, several enzymes. These enzymes include those needed for the biogenesis of purine bases; for the transfer of desoxyuridine to thymidine; and for the biosynthesis of methionine, glycine, and formylmethionyl-transfer-RNA. This results in suppression of protein synthesis, impairment of metabolic processes, and inhibition of growth and multiplication of those organisms that cannot use preformed folate. The effect is bacteriostatic, although a bactericidal action is evident at the high concentrations that may be found in urine.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2076