

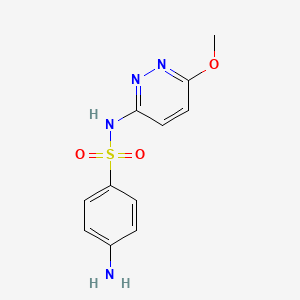

1. Sulfapyridazine
2. Sulphamethoxypyridazine
1. 80-35-3
2. Sulphamethoxypyridazine
3. Midicel
4. Sulfapyridazine
5. Depovernil
6. Sulfalex
7. Sulfametoxipiridazine
8. Spofadazine
9. Sulfdurazin
10. Lederkyn
11. Longin
12. 3-sulfa-6-methoxypyridazine
13. Sulfamethoxypyridazinum
14. Lisulfen
15. Petrisul
16. Piridolo
17. Quinoseptyl
18. Retasulfin
19. Sulfametoxipiridazina
20. Sulfozona
21. Sultirene
22. Altezol
23. Davosin
24. Lentac
25. Medicel
26. Midikel
27. Myasul
28. Opinsul
29. Paramid
30. Retamid
31. Slosul
32. Vinces
33. Durox
34. Kinex
35. Kynex
36. Smop
37. Solfametossipiridazina
38. Paramid Supra
39. 3-methoxy-6-sulfanylamidopyridazine
40. 3-sulfanilamide-6-methoxypyridazine
41. 3-sulfanilamido-6-methoxypyridazine
42. 6-methoxy-3-sulfanilamidopyridazine
43. 6-sulfanilamido-3-methoxypyridazine
44. 4-amino-n-(6-methoxypyridazin-3-yl)benzenesulfonamide
45. Retasulphine
46. 3-(p-aminobenzenesulfamido)-6-methoxypyridazine
47. 3-p-aminobenzenesulphonamido-6-methoxypyridazine
48. Benzenesulfonamide, 4-amino-n-(6-methoxy-3-pyridazinyl)-
49. Sulfamethoxipyridazine
50. 4-amino-n-(6-methoxy-3-pyridazinyl)benzenesulfonamide
51. 4-amino-n-(6-methoxy-3-pyridazinyl)-benzenesulfonamide
52. Cl 13494
53. 6-methoxy-3-pyridazinylsulfanilamide
54. Sulfamethoxypyridazine [inn]
55. Rp 7522
56. Chebi:102516
57. 3-(4-aminobenzenesulfonamido)-6-methoxypyridazine
58. N(sup 1)-(6-methoxy-3-pyridazinyl)sulfanilamide
59. Nsc-757875
60. Mls000069641
61. T034e4ns2z
62. N(1)-(6-methoxy-3-pyridazinyl)sulfanilamide
63. Ncgc00016324-01
64. Cl-13494
65. N-(6-methoxy-3-pyridazinyl)sulfanilamide
66. Smr000018386
67. Sulfamethoxipyridazinum
68. Dsstox_cid_3611
69. Dsstox_rid_77108
70. Dsstox_gsid_23611
71. Sulfamethoxypyridazine (inn)
72. Sulfamethoxypyridazine 100 Microg/ml In Acetonitrile
73. Cas-80-35-3
74. Solfametossipiridazina [dcit]
75. Sr-01000000179
76. Sulfamethoxypyridazinum [inn-latin]
77. Sulfametoxipiridazina [inn-spanish]
78. Einecs 201-272-5
79. Brn 0277076
80. Unii-t034e4ns2z
81. Kineks
82. N1-(6-methoxy-3-pyridazinyl)sulfanilamide
83. Lederkyn (tn)
84. Prestwick_118
85. 4-amino-n-(6-methoxy-3-pyridazinyl)benzolsulfonamid
86. Sulfamethoxypyridazine [usp:inn:ban]
87. Spectrum_001151
88. Opera_id_698
89. Prestwick0_000724
90. Prestwick1_000724
91. Prestwick2_000724
92. Prestwick3_000724
93. Pyridazine, Sulfamethoxy-
94. Spectrum2_001429
95. Spectrum3_001462
96. Spectrum4_000430
97. Spectrum5_001187
98. Epitope Id:122239
99. Sulphamethazine Sodium Salt
100. Oprea1_275757
101. Schembl93617
102. Bspbio_000648
103. Bspbio_002983
104. Kbiogr_000760
105. Kbioss_001631
106. 5-25-12-00424 (beilstein Handbook Reference)
107. Mls000100719
108. Mls001148433
109. Divk1c_000239
110. Spectrum1501156
111. Spbio_001538
112. Spbio_002587
113. Bpbio1_000714
114. Chembl268869
115. Sulfanilamide, N(sup 1)-(6-methoxy-3-pyridazinyl)-
116. 4-amino-n-(6-methoxy-pyridazin-3-yl)-benzenesulfonamide
117. Benzenesulfonamide,4-amino-n-(6-methoxy-3-pyridazinyl)-
118. Dtxsid5023611
119. Hms500l21
120. Kbio1_000239
121. Kbio2_001631
122. Kbio2_004199
123. Kbio2_006767
124. Kbio3_002483
125. Zinc49141
126. Ninds_000239
127. Hms1570a10
128. Hms1921l17
129. Hms2092j05
130. Hms2097a10
131. Hms2234f17
132. Hms3652h21
133. Hms3714a10
134. Pharmakon1600-01501156
135. Sulfamethoxypyridazine [mi]
136. 3-methoxy-6-sulfanilamidopyridazine
137. Hy-b1387
138. Tox21_110373
139. Ccg-38976
140. Mfcd00057372
141. Nsc757875
142. S4250
143. Sulfamethoxypyridazine [mart.]
144. Akos000605846
145. Tox21_110373_1
146. Cs-4821
147. Db13773
148. Nsc 757875
149. Sdccgmls-0003277.p003
150. Sulfamethoxypyridazine [who-dd]
151. Sulfamethoxypyridazine [who-ip]
152. Idi1_000239
153. Ncgc00016324-02
154. Ncgc00016324-03
155. Ncgc00016324-04
156. Ncgc00016324-05
157. Ncgc00016324-06
158. Ncgc00016324-08
159. Ncgc00016324-09
160. Ncgc00022232-03
161. Ncgc00022232-04
162. Ac-12005
163. Ac-32613
164. Bs-32820
165. Sbi-0051666.p002
166. Sulfamethoxypyridazine, Analytical Standard
167. Ab00052228
168. Ft-0653623
169. S0591
170. Sw196429-3
171. Sulfamethoxypyridazinum [who-ip Latin]
172. D02439
173. D70409
174. Sulfanilamide, N1-(6-methoxy-3-pyridazinyl)-
175. Ab00052228_12
176. Sulfamethoxypyridazine 100 Microg/ml In Methanol
177. A839894
178. Sulfamethoxypyridazine 1000 Microg/ml In Methanol
179. Q7636177
180. Sr-01000000179-3
181. Sr-01000000179-4
182. W-104237
183. Brd-k00938507-001-05-3
184. Brd-k00938507-001-13-7
185. 4-amino-n-[6-(methyloxy)pyridazin-3-yl]benzenesulfonamide
186. Sulfamethoxypyridazine, Vetranal(tm), Analytical Standard
187. Sulfamethoxypyridazine, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 280.31 g/mol |
|---|---|
| Molecular Formula | C11H12N4O3S |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 280.06301143 g/mol |
| Monoisotopic Mass | 280.06301143 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 376 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01ED - Long-acting sulfonamides
J01ED05 - Sulfamethoxypyridazine