


1. Mebacid
2. Methylsulfadiazine
3. Trimetox
1. 127-79-7
2. Sulphamerazine
3. Sulfamerazin
4. Sulfamethyldiazine
5. Cremomerazine
6. Methylsulfazine
7. Sulfameradine
8. Mebacid
9. Mesulfa
10. Metilsulfadiazin
11. 2-sulfa-4-methylpyrimidine
12. 4-amino-n-(4-methylpyrimidin-2-yl)benzenesulfonamide
13. Methylpyrimal
14. Metilsulfazin
15. Sulfamerazina
16. Sulfamerazinum
17. Kelamerazine
18. Percoccide
19. Pyralcid
20. Septacil
21. Septosyl
22. Solumedin
23. Sumedine
24. Romezin
25. Veta-merazine
26. Debenal-m
27. Pirimal-m
28. Pyrimal M
29. Methylsulfazin
30. Benzenesulfonamide, 4-amino-n-(4-methyl-2-pyrimidinyl)-
31. N-(4-methyl-2-pyrimidyl)sulfanilamide
32. 2-(sulfanilamido)-4-methylpyrimidine
33. 2-(4-aminobenzenesulfonamido)-4-methylpyrimidine
34. 4-amino-n-(4-methyl-2-pyrimidinyl)-benzenesulfonamide
35. 2643-rp
36. Rp 2632
37. N1-(4-methyl-2-pyrimidinyl)sulfanilamide
38. (p-aminobenzolsulfonyl)-2-amino-4-methylpyrimidin
39. A-310
40. 4-amino-n-(4-methyl-2-pyrimidinyl)benzenesulfonamide
41. Nsc 27259
42. Rp2632
43. Sulfanilamide, N1-(4-methyl-2-pyrimidinyl)-
44. Solumedine
45. Sulfamerazine (inn)
46. Chebi:102130
47. 2-sulfanilamido-4-methylpyrimidine
48. 2632 R. P.
49. N(1)-(4-methyl-2-pyrimidinyl)sulfanilamide
50. 2-(p-aminobenzolsulfonamido)-4-methylpyrimidine
51. Nsc-27259
52. Ur1sab295f
53. Mls000551747
54. Solfamerazina
55. Sulfanilamide, N(sup 1)-(4-methyl-2-pyrimidinyl)-
56. Ncgc00016386-01
57. Cas-127-79-7
58. Smr000145672
59. Solfamerazina [dcit]
60. Sulfamerazine 100 Microg/ml In Acetonitrile
61. Dsstox_cid_3612
62. Sulfamerazine [inn]
63. Dsstox_rid_77109
64. Dsstox_gsid_23612
65. Sulfamerazinum [inn-latin]
66. Sulfamerazina [inn-spanish]
67. 4-amino-n-(4-methyl-pyrimidin-2-yl)-benzenesulfonamide
68. 4-amino-n-(4-methylpyrimidin-2-yl)
69. Sr-01000684857
70. Einecs 204-866-2
71. N(sup 1)-(4-methyl-2-pyrimidinyl)sulfanilamide
72. Unii-ur1sab295f
73. Brn 0249133
74. 4-amino-n-(4-methylpyrimidin-2-yl)benzene-1-sulfonamide
75. Ai3-08026
76. Sulfamerazine [usp:inn:ban]
77. (p-aminobenzolsulfonyl)-2-amino-4-methylpyrimidin [german]
78. Prestwick_17
79. Mfcd00023212
80. N,n-dialkylarylamine
81. Sulfamerazine-13c6
82. Spectrum_000003
83. N1-(4-methylpyrimidin-2-yl)sulfanilamide
84. Opera_id_988
85. Prestwick0_000694
86. Prestwick1_000694
87. Prestwick2_000694
88. Prestwick3_000694
89. Spectrum2_001320
90. Spectrum3_001363
91. Spectrum4_000343
92. Spectrum5_001413
93. 2(p-aminobenzolsulfonamido)-4-methylpyrimidin
94. Sulfamerazine [mi]
95. Chembl438
96. Epitope Id:122236
97. N(sup1)-(4-methyl-2-pyrimidinyl)sulfanilamide
98. Schembl33999
99. Bspbio_000847
100. Bspbio_002886
101. Kbiogr_000745
102. Kbioss_000343
103. Sulfamerazine [vandf]
104. 5-25-10-00167 (beilstein Handbook Reference)
105. Mls001201765
106. Divk1c_000563
107. Spectrum1500547
108. Sulfamerazine [mart.]
109. Spbio_001419
110. Spbio_002768
111. Sulfamerazine [usp-rs]
112. Sulfamerazine [who-dd]
113. Bpbio1_000933
114. Dtxsid0023612
115. Hms501m05
116. Kbio1_000563
117. Kbio2_000343
118. Kbio2_002911
119. Kbio2_005479
120. Kbio3_002106
121. Zinc57501
122. Ninds_000563
123. Hms1570k09
124. Hms1921a15
125. Hms2092i17
126. Hms2097k09
127. Hms2234d16
128. Hms3374k04
129. Hms3652i03
130. Hms3714k09
131. Pharmakon1600-01500547
132. Sulfamerazine [green Book]
133. Sulfamerazine [orange Book]
134. Albb-025702
135. Amy23374
136. Hy-b0512
137. Nsc27259
138. Sulfamerazine [ep Monograph]
139. Tox21_110411
140. Bbl003544
141. Ccg-39258
142. Nsc757325
143. S3132
144. Stk520614
145. Sulfose Component Sulfamerazine
146. Akos005143010
147. Terfonyl Component Sulfamerazine
148. Tox21_110411_1
149. Db01581
150. Ks-5323
151. Nsc-757325
152. Idi1_000563
153. Lantrisul Component Sulfamerazine
154. Sulfaloid Component Sulfamerazine
155. Ncgc00016386-02
156. Ncgc00016386-03
157. Ncgc00016386-06
158. Ncgc00094787-01
159. Ncgc00094787-02
160. Neotrizine Component Sulfamerazine
161. Sulfamerazine 100 Microg/ml In Methanol
162. Sulfamerazine Component Of Sulfose
163. N'-(4-methyl-2-pyrimidyl) Sulfanilamide
164. Sulfamerazine (trisulfapyrimidines)
165. Sulfamerazine Component Of Terfonyl
166. Trisulfapyrimidines (sulfamerazine)
167. Sbi-0051521.p003
168. Sulfamerazine 1000 Microg/ml In Methanol
169. Sulfamerazine Component Of Lantrisul
170. Sulfamerazine Component Of Sulfaloid
171. Db-041873
172. Sulfamerazine Component Of Neotrizine
173. Sulfamerazine, Reagentplus(r), >=99.0%
174. Ab00052096
175. Ft-0631745
176. Ft-0645132
177. Sw196334-3
178. Triple Sulfoid Component Sulfamerazine
179. Sulfadimidine Impurity A [ep Impurity]
180. Sulfamerazine, Vetec(tm) Reagent Grade, 98%
181. 2-(p-aminobenzosulfonamido)-4-methylpyrimidine
182. D02435
183. D84140
184. Ab00052096-13
185. Ab00052096_15
186. Ab00052096_16
187. Sulfamerazine Component Of Triple Sulfoid
188. A805747
189. Q415196
190. Sulfamerazine, Vetranal(tm), Analytical Standard
191. Sulfonamides Duplex Component Sulfamerazine
192. Q-201761
193. Sr-01000684857-2
194. Sr-01000684857-4
195. Brd-k93524252-001-05-6
196. Brd-k93524252-001-15-5
197. Sulfamerazine Component Of Sulfonamides Duplex
198. F2190-0484
199. N(sup 1)-(4-methyl-2- Pyrimidinyl)sulfanilamide
200. Trisulfapyrimidines (sulfamerazine) [orange Book]
201. Sulfamerazine, European Pharmacopoeia (ep) Reference Standard
202. Sulfamerazine, United States Pharmacopeia (usp) Reference Standard
203. Sulfamerazine, Pharmaceutical Secondary Standard; Certified Reference Material
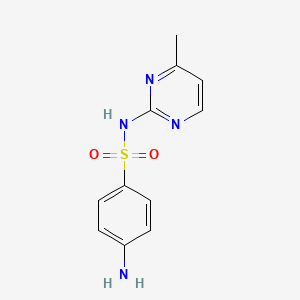
| Molecular Weight | 264.31 g/mol |
|---|---|
| Molecular Formula | C11H12N4O2S |
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 264.06809681 g/mol |
| Monoisotopic Mass | 264.06809681 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 360 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A sulfanilamide that is used as an antibacterial agent. It can be used to treat bronchitis, prostatitis and urinary tract infections.
Sulfonamides act as competitive inhibitors of the enzyme dihydropteroate synthetase (DHPS), an enzyme involved in folate synthesis in bacteria.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BA - Sulfonamides
D06BA06 - Sulfamerazine
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01ED - Long-acting sulfonamides
J01ED07 - Sulfamerazine
Absorption
Rapidly absorbed following oral administration.
Sulfamerazine has known human metabolites that include N(4)-Acetylsulfamerazine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Sulfamerazine is a sulfonamide drug that inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid (PABA) for binding to dihydropteroate synthetase (dihydrofolate synthetase). Sulfamerazine is bacteriostatic in nature. Inhibition of dihydrofolic acid synthesis decreases the synthesis of bacterial nucleotides and DNA.

LOOKING FOR A SUPPLIER?