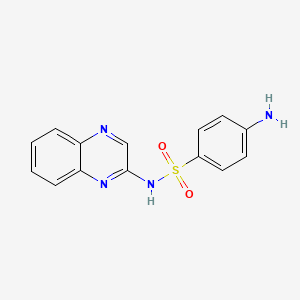

1. Sulfabenzpyrazine
2. Sulfachinoxalin
1. 59-40-5
2. Sulfabenzpyrazine
3. Sulphaquinoxaline
4. Avicocid
5. Sulfaline
6. Sulquin
7. Italquina
8. Kokozigal
9. Sulfacox
10. Ursokoxaline
11. 2-sulfanilamidoquinoxaline
12. 2-sulfanilamidobenzopyrazine
13. Sulfa-q 20
14. Sulfachinoxalin
15. Sulfaquinoxalin
16. 4-amino-n-(quinoxalin-2-yl)benzenesulfonamide
17. Sulfaquinoxalina
18. 2-(p-sulfanilamido)quinoxaline
19. Sulfaquinoxalinum
20. Compound 3-120
21. Anti-k
22. N-(2-quinoxalinyl)sulfanilamide
23. N1-(2-quinoxalinyl)sulfanilamide
24. N'-2-quinoxalylsulfanilamide
25. Benzenesulfonamide, 4-amino-n-2-quinoxalinyl-
26. 2-p-aminobenzenesulfonamidoquinoxaline
27. 4-amino-n-quinoxalin-2-ylbenzenesulfonamide
28. Sq 40
29. N-(2-quinoxalinyl)sulfanilide
30. 2-p-aminobenzenesulphonamidoquinoxaline
31. Nsc41805
32. Nsc-41805
33. N(sup 1)-2-quinoxalinylsulfanilamide
34. N(sup 1)-(2-quinoxalinyl)sulfanilamide
35. Sqx
36. Ai3-17254
37. 4-amino-n-(2-quinoxalinyl)benzenesulfonamide
38. Sulfanilamide, N1-2-quinoxalinyl-
39. Sulquin 6-50 Concentrate (veterinary)
40. Sulfaquinoxaline (usp/inn)
41. Mls000737045
42. 4-amino-n-(quinoxalin-2-yl)benzene-1-sulfonamide
43. Wnw8115tm9
44. N'-(2-quinoxalyl)sulfanilamide
45. N1-2-quinoxalinyl, Sulfanilamide
46. N(sup1)-quinoxalin-2-ylsulfanilamide
47. N(sup1)-(2-quinoxalinyl)sulfanilamide
48. 2-(p-aminobenzene)sulfonamidoquinoxaline
49. Dsstox_cid_22424
50. Dsstox_rid_80021
51. Dsstox_gsid_42424
52. 4-amino-n-quinoxalin-2-yl-benzenesulfonamide
53. Caswell No. 721
54. Aviochina
55. Sulfaquinoxaline, Analytical Standard
56. 4-amino-n-2-quinoxalinylbenzenesulfonamide
57. Sul-q-nox
58. Cas-59-40-5
59. Nsc 41805
60. Smr000394001
61. Sulfaquinoxalinum [inn-latin]
62. Sulfaquinoxalina [inn-spanish]
63. Hsdb 7440
64. Sulfaquinoxaline [usan:inn:ban]
65. Einecs 200-423-2
66. Epa Pesticide Chemical Code 077901
67. Brn 0290026
68. Unii-wnw8115tm9
69. Embazin
70. Nococcin
71. Quinoxipra C
72. Kokozigal S
73. Sulfa-q
74. Sulfaquinoxaline [usp:inn:ban]
75. Spectrum_001410
76. Sulfaquinoxaline-13c6
77. Sulfanilamide, N(sup 1)-2-quinoxalinyl-
78. 4-amino-n-2-quinoxalinyl-benzenesulfonamide
79. Prestwick0_000731
80. Prestwick1_000731
81. Prestwick2_000731
82. Prestwick3_000731
83. Spectrum2_000986
84. Spectrum3_001455
85. Spectrum4_000423
86. Spectrum5_000983
87. S. Q. 40 Per Cent
88. Ncistruc1_000587
89. Ncistruc2_000454
90. Oprea1_705564
91. Schembl93789
92. Sulfaquinoxaline, Ban, Inn
93. Bspbio_000682
94. Bspbio_002949
95. Kbiogr_000746
96. Kbioss_001890
97. Sulfaquinoxaline [mi]
98. 5-25-11-00125 (beilstein Handbook Reference)
99. Mls001176103
100. S. Q. "40 Per Cent"
101. S. Q. '40 Per Cent'
102. Bidd:gt0642
103. Divk1c_000035
104. Sulfaquinoxaline [inn]
105. Spbio_001051
106. Spbio_002621
107. Sulfaquinoxaline [hsdb]
108. Bpbio1_000752
109. Zinc2106
110. Chembl1437847
111. Dtxsid8042424
112. N1-2-quinoxalinyl-sulfanilamide
113. Schembl21295162
114. Sulfaquinoxaline [mart.]
115. Chebi:94719
116. Kbio1_000035
117. Kbio2_001890
118. Kbio2_004458
119. Kbio2_007026
120. Kbio3_002449
121. Ninds_000035
122. Hms2744n03
123. Albb-033368
124. Hy-b1282
125. Nci41805
126. Str08438
127. Wln: T66 Bn Enj Cmswr Dz
128. Tox21_110027
129. Tox21_300894
130. Ccg-38184
131. Mfcd00055406
132. Ncgc00013489
133. Sulfaquinoxaline [green Book]
134. N(sup1)-2-quinoxalinyl-sulfanilamide
135. Akos001053134
136. Akos024418782
137. Tox21_110027_1
138. Db11464
139. N(sup 1)-2-quinoxalinyl-sulfanilamide
140. Sulfaquinoxaline [usp Impurity]
141. Idi1_000035
142. Smp2_000015
143. Sulfaquinoxaline [usp Monograph]
144. Ncgc00013489-01
145. Ncgc00013489-02
146. Ncgc00013489-03
147. Ncgc00096603-01
148. Ncgc00178376-05
149. Ncgc00254798-01
150. Sulfanilamide, N(sup1)-2-quinoxalinyl-
151. Ac-18848
152. Nci60_003947
153. Sulfanilamide, N1-2-quinoxalinyl- (8ci)
154. Sulfaquinoxalin 100 Microg/ml In Methanol
155. Db-053379
156. Cs-0013058
157. Ft-0631264
158. En300-24981
159. Sulfaquinoxalin 100 Microg/ml In Acetonitrile
160. D05952
161. H11037
162. 4-amino-n-2-quinoxalinylbenzenesulfonamide, 9ci
163. 055s406
164. A928697
165. Sr-01000768431
166. Q1019320
167. Sr-01000768431-3
168. Sulfaquinoxaline, Pestanal(r), Analytical Standard
169. W-105325
170. Brd-k71133585-236-05-2
171. Brd-k71133585-236-08-6
172. Z56922081
173. Sulfaquinoxaline, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 300.34 g/mol |
|---|---|
| Molecular Formula | C14H12N4O2S |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 300.06809681 g/mol |
| Monoisotopic Mass | 300.06809681 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 442 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Mesh Heading: anti-infective agents, Antiprotozoal agents
National Library of Medicine, SIS; ChemIDplus Record for Sulfaquinoxaline (59-40-5). Available from, as of April 13, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
MEDICATION (VET): Sulfonamide antibiotic. Also used as a coccidiostat in poultry.
Milne, G.W.A. Veterinary Drugs: Synonyms and Properties. Ashgate Publishing Limited, Aldershot, Hampshire, England 2002., p. 64
MEDICATION (VET): Sulfonamides /including sulfaquinoxaline/ are widely used for treatment of several bacterial and protozoal infections in poultry.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2261
MEDICATION (VET): ... Used to treat or control outbreaks of coccidiosis caused by Eimeria tenella, E. necatrix, E. acervulina, E. maxima, or E. brunetti in chickens; by E. meleagrimitis or E. adenoeides in turkeys; and by E. bovis or E. zurnii in cattle. It is also used to treat or control fowl cholera caused by Pasteurella multocida, as well as fowl typhoid caused by sensitive organisms.
Ullmann's Encyclopedia of Industrial Chemistry. 6th ed.Vol 1: Federal Republic of Germany: Wiley-VCH Verlag GmbH & Co. 2003 to Present, p. V. 38 24 (2003)
For more Therapeutic Uses (Complete) data for SULFAQUINOXALINE (14 total), please visit the HSDB record page.
(VET): Prolonged administration of sulfaquinoxaline may result in deposition of crystals in the kidney or interference with normal blood clotting. Sulfaquinoxaline levels of greater than 0.012% in drinking water for more than twenty-four to thirty-six hours may result in reduced growth rate from decreased feed or water consumption.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Sulfonamides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
(VET): Animals should maintain an adequate water intake during the treatment period.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Sulfonamides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
(VET): An idiosyncratic sulfonamide toxicosis can occur in any breed of dog, but has been reported more frequently in the Doberman Pinscher than in other breeds. This specific type of drug reaction includes blood dyscrasias, nonseptic polyarthritis, and skin rash. Dogs given sulfonamides may also develop cutaneous eruptions, hepatitis, or keratitis sicca. Dogs are reported to develop a hemorrhagic syndrome when doses of sulfaquinoxaline that are tolerated by many chickens are administered in their drinking water.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Sulfonamides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
(VET): Clotting disorders similar to those resulting from coumarin anticoagulants have been reported in chickens and dogs.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Sulfonamides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
For more Drug Warnings (Complete) data for SULFAQUINOXALINE (11 total), please visit the HSDB record page.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
Sulfaquinoxaline is minimally absorbed systemically and is referred to as an enteric sulfonamide.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Sulfonamides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
Sulfonamides are distributed into milk; however, the sulfonamides that are clinically relevant to food-producing animals are distributed into milk in concentrations too low to be therapeutic but high enough to produce residues. Sulfadiazine and sulfanilamide are more efficiently distributed into milk than most sulfonamides, but are not used in dairy cattle. For many sulfonamides, 0.5 to 2% of the total dose is found in the milk. Distribution into milk varies depending on the amount of non-protein -bound sulfonamide present in the blood and the amount of the nonionized and therefore liposoluble form of the medication present. Sulfonamides with higher pKa values produce a higher proportion of drug in the blood that is non-ionized, and if other factors, such as the rate of biotransformation, also support it, may be distributed more easily into milk. /Sulfonamides/
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Sulfonamides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
Sulfonamides are eliminated from body partly as unchanged drugs and partly as metabolic products. The largest fraction is excreted in urine, and half-life ... is thus dependent on renal function. In acid urine, the older sulfonamides are insoluble and may precipitate, causing crystalline deposits that can cause urinary obstruction. Small amt are eliminated in feces and in bile, milk, and other secretions. /Sulfonamides/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1174
All sulfonamides are bound in varying degree to plasma proteins, particularly to albumin. The extent ... Is determined by the hydrophobicity and ... pKa; at physiological pH, drugs with a high pKa exhibit a low degree of protein binding ... /They/ are distributed throughout all tissues of the body ... /and/ readily enter pleural, peritoneal, synovial, ocular, and similar body fluids ... in the unbound active form. /Sulfonamides/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1173
For more Absorption, Distribution and Excretion (Complete) data for SULFAQUINOXALINE (8 total), please visit the HSDB record page.
Sulfonamides are primarily metabolized in the liver but metabolism also occurs in other tissues. Biotransformation occurs mainly by acetylation, glucuronide conjugation, and aromatic hydroxylation in many species. The types of metabolites formed and the amount of each varies depending on the specific sulfonamide administered; the species, age, diet, and environment of the animal; the presence of disease; and, with the exception of pigs and ruminants, even the sex of the animal. Dogs are considered to be unable to acetylate sulfonamides to any significant degree. /Sulfonamides/
The sulfonamides undergo metabolic alterations in vivo, especially in the liver. The major metabolic derivative is N4-acetylated sulfonamide. Acetylation, which occurs to a different extent with each agent, is disadvantageous, because the resulting products have no antibacterial activity and yet retain the toxic potentialities of the parent substance. /Sulfonamides/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1173
Bacteriostatic. Sulfonamides interfere with the biosynthesis of folic acid in bacterial cells; they compete with paraaminobenzoic acid (PABA) for incorporation in the folic acid molecule. By replacing the PABA molecule and preventing the folic acid formation required for DNA synthesis, the sulfonamides prevent multiplication of the bacterial cell. Susceptible organisms must synthesize their own folic acid; mammalian cells use preformed folic acid and, therefore, are not susceptible. Cells that produce excess PABA or environments with PABA, such as necrotic tissues, allow for resistance by competition with the sulfonamide. /Sulfonamides/
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Sulfonamides (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
Sulfonamides are structural analogs and competitive antagonists of para-aminobenzoic acid (PABA) and thus prevent normal bacterial utilization of PABA for the synthesis of folic acid (pteroylglutamic acid). More specifically, sulfonamides are competitive inhibitors of dihydropteroate synthase,the bacterial enzyme responsible for the incorporation of PABA into dihydropteroic acid, the immediate precursor of folic acid. Sensitive microorganisms are those that must synthesize their own folic acid; bacteria that can utilize preformed folate are not affected. Bacteriostasis induced by sulfonamides is counteracted by PABA competitively. Sulfonamides do not affect mammalian cells by this mechanism, since they require preformed folic acid and cannot synthesize it. /Sulfonamides/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1172