
1. Anturan
2. Anturane
3. Apo Sulfinpyrazone
4. Apo-sulfinpyrazone
5. Nu Sulfinpyrazone
6. Nu-sulfinpyrazone
7. Sulfoxyphenylpyrazolidin
8. Sulphinpyrazone
1. 57-96-5
2. Sulphinpyrazone
3. Anturane
4. (+/-)-sulfinpyrazone
5. Sulfinpyrazon
6. Anturan
7. Diphenylpyrazone
8. Sulfoxyphenylpyrazolidine
9. Anturanil
10. Anturidin
11. Enturen
12. Anturano
13. Sulfinpyrazine
14. Usaf Ge-13
15. 1,2-diphenyl-4-(2-(phenylsulfinyl)ethyl)pyrazolidine-3,5-dione
16. Sulfinpyrazonum
17. Anturen
18. Enturan
19. Nsc 75925
20. 4-[2-(benzenesulfinyl)ethyl]-1,2-diphenylpyrazolidine-3,5-dione
21. Anturane (tn)
22. G 28315
23. 4-(phenylsulfoxyethyl)-1,2-diphenyl-3,5-pyrazolidinedione
24. 1,2-diphenyl-4-(2'-phenylsulfinethyl)-3,5-pyrazolidinedione
25. Chebi:9342
26. 1,2-diphenyl-3,5-dioxo-4-(2-phenylsulfinylethyl)pyrazolidine
27. 4-(2-benzenesulfinylethyl)-1,2-diphenylpyrazolidine-3,5-dione
28. 3,5-pyrazolidinedione, 1,2-diphenyl-4-(2-(phenylsulfinyl)ethyl)-
29. Nsc-75925
30. 1,2-diphenyl-4-(2-(phenylsulfinyl)ethyl)-3,5-pyrazolidinedione
31. G-28315
32. Diphenylpyrazone;g-28315
33. V6ofu47k3w
34. ( Inverted Exclamation Marka)-sulfinpyrazone
35. 1,2-diphenyl-4-[2-(phenylsulfinyl)ethyl]-3,5-pyrazolidinedione
36. 1,2-diphenyl-3,5-dioxo-4-(2'-phenyl-sulfinyl-aethyl)-pyrazolidin
37. 3,5-pyrazolidinedione, 1,2-diphenyl-4-[2-(phenylsulfinyl)ethyl]-
38. Nsc75925
39. Apo-sulfinpyrazone
40. Cas-57-96-5
41. Ncgc00016255-01
42. Sulfinpirazona
43. 1,2-diphenyl-4-[2-(phenylsulfinyl)ethyl]pyrazolidine-3,5-dione
44. Dsstox_cid_3618
45. Dsstox_rid_77113
46. Dsstox_gsid_23618
47. Sulfinpyrazonum [inn-latin]
48. Sulfinpirazona [inn-spanish]
49. Smr000058991
50. Sulfinpyrazone (spz)
51. Hsdb 3396
52. Sr-01000003152
53. Einecs 200-357-4
54. Unii-v6ofu47k3w
55. Brn 0713597
56. G 28,315
57. Sufinpyrazone
58. Suphinpyrazone
59. Sulphinepyrazolone
60. Prestwick_455
61. 1,2-diphenyl-3,5-dioxo-4-(2'-phenyl-sulfinyl-aethyl)-pyrazolidin [german]
62. Sulfinpyrazone [usp:inn:ban:jan]
63. (-+)-sulfinpyrazone
64. Spectrum_001022
65. Prestwick0_000290
66. Prestwick1_000290
67. Prestwick2_000290
68. Prestwick3_000290
69. Spectrum2_001324
70. Spectrum3_000590
71. Spectrum4_000845
72. Spectrum5_000984
73. Sulfinpyrazone [mi]
74. Sulfinpyrazone [inn]
75. Sulfinpyrazone [jan]
76. Bidd:pxr0096
77. Schembl34421
78. Bspbio_000200
79. Bspbio_002040
80. Kbiogr_001449
81. Kbioss_001502
82. Mls000028565
83. Mls001074941
84. Divk1c_000090
85. Spectrum1500554
86. Sulfinpyrazone [vandf]
87. Spbio_001447
88. Spbio_002419
89. Sulfinpyrazone [mart.]
90. Bpbio1_000220
91. Gtpl5826
92. Sulfinpyrazone (jan/usp/inn)
93. Sulfinpyrazone [usp-rs]
94. Sulfinpyrazone [who-dd]
95. Dtxsid0023618
96. Hms500e12
97. Kbio1_000090
98. Kbio2_001502
99. Kbio2_004070
100. Kbio2_006638
101. Kbio3_001540
102. Ninds_000090
103. 1,2-diphenyl-4-(phenylsulfinylethyl)-3,5-pyrazolidinedione
104. Hms1568j22
105. Hms1921c09
106. Hms2092k11
107. Hms2095j22
108. Hms2233h11
109. Hms3259i06
110. Hms3371b22
111. Hms3712j22
112. Hms3885f12
113. Pharmakon1600-01500554
114. Bcp05344
115. Hy-b1271
116. Sulfinpyrazone [ep Impurity]
117. Sulfinpyrazone [orange Book]
118. Sulfinpyrazone For System Suitability
119. Tox21_110331
120. 4-[2-(benzenesulfinyl)ethyl]-1,2-diphenyl-pyrazolidine-3,5-dione
121. Bdbm50237626
122. Ccg-39262
123. Mfcd00057279
124. Nsc757332
125. S4628
126. Sulfinpyrazone [ep Monograph]
127. Sulfinpyrazone [usp Impurity]
128. Akos015913283
129. Tox21_110331_1
130. Db01138
131. Ds-6609
132. Nc00539
133. Nsc-757332
134. Idi1_000090
135. (invertedexclamationmarka)-sulfinpyrazone
136. Ncgc00016255-02
137. Ncgc00016255-03
138. Ncgc00016255-04
139. Ncgc00016255-05
140. Ncgc00016255-06
141. Ncgc00016255-07
142. Ncgc00016255-10
143. Ncgc00018267-01
144. Ncgc00023932-03
145. Ncgc00023932-04
146. Ac-13602
147. Ac-32767
148. (+/-)-sulfinpyrazone, Analytical Standard
149. Sbi-0051528.p003
150. Wln: T5vnnv Ehj Br& Cr& E2so&r
151. Db-053135
152. Ab00052103
153. Cs-0013052
154. Ft-0603240
155. U0114
156. 4-(phenylsulfoxyethyl)-1,5-pyrazolidinedione
157. C07317
158. D00449
159. H10364
160. 3, 1,2-diphenyl-4-[2-(phenylsulfinyl)ethyl]-
161. Ab00052103_13
162. A869550
163. Q3790542
164. Sr-01000003152-2
165. Sr-01000003152-4
166. W-105427
167. Brd-a36217750-001-05-6
168. Brd-a36217750-001-09-8
169. Z1565440323
170. Sulfinpyrazone, European Pharmacopoeia (ep) Reference Standard
171. 1,2-di(phenyl)-4-(2-phenylsulfinylethyl)pyrazolidine-3,5-dione
172. 1,2-diphenyl-4-[2-(phenylsulfinyl)-ethyl]-pyrazolidine-3,5-dione
173. Sulfinpyrazone, United States Pharmacopeia (usp) Reference Standard
174. Sulfinpyrazone For System Suitability, European Pharmacopoeia (ep) Reference Standard
175. Sulfinpyrazone; 1,2-diphenyl-4-(2-phenylsulphinylethyl)pyrazolidine-3,5-dione;(+/-)-sulfinpyrazone
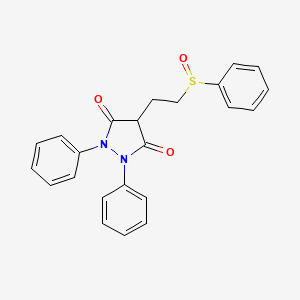
| Molecular Weight | 404.5 g/mol |
|---|---|
| Molecular Formula | C23H20N2O3S |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 404.11946368 g/mol |
| Monoisotopic Mass | 404.11946368 g/mol |
| Topological Polar Surface Area | 76.9 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 571 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Uricosuric Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
EXPTL USE: SULFINPYRAZONE...PROLONGS PLATELET SURVIVAL IN MAN. ...BLOCK PLATELET AGGREGATION IN RESPONSE TO COLLAGEN & ANTIGEN-ANTIBODY COMPLEXES BUT NOT IN RESPONSE TO ADP OR THROMBIN...APPEARS RELATED TO DIMINISHED RELEASE OF ADP & 5-HYDROXYTRYPTAMINE. ...BEING STUDIED FOR...PROPHYLACTIVE VALUE IN THROMBOEMBOLIC DISORDERS
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1365
SULFINPYRAZONE USP...AS OFFICIAL 100-MG TABLETS & 200 MG CAPSULES. FOR... CHRONIC GOUT, INITIAL DOSAGE IS 100-200 MG/DAY. AFTER 1ST WK, DOSE...GRADUALLY INCR UNTIL SATISFACTORY LOWERING OF PLASMA URIC ACID IS ACHIEVED & MAINTAINED...MAY REQUIRE FROM 100-400 MG/DAY, DIVIDED INTO 2-4 DOSES & PREFERABLY GIVEN WITH MEALS.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 864
OCCASIONAL RESISTANT PT HAVE BEEN TREATED SUCCESSFULLY WITH DOSES AS HIGH AS 800 MG/DAY. LARGER DOSES ARE POORLY TOLERATED & UNLIKELY TO PRODUCE FURTHER URICOSURIC EFFECT IN RESISTANT PT. IN RESPONSIVE PT...SINGLE DAILY DOSE OF 100 MG IS SOMETIMES SATISFACTORY FOR MAINTENANCE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 864
For more Therapeutic Uses (Complete) data for SULFINPYRAZONE (10 total), please visit the HSDB record page.
SULFINPYRAZONE LACKS CLINICALLY-STRIKING ANTI-INFLAMMATORY & ANALGESIC PROPERTIES OF ITS CONGENER, PHENYLBUTAZONE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 864
GI IRRITATION OCCURS IN 10-15% OF ALL PT...AN OCCASIONAL PT MAY REQUIRE DISCONTINUANCE OF ITS USE. FREQUENCY & SEVERITY INCR WITH DOSAGE...DISTRESS IS LESSENED WHEN DRUG IS TAKEN IN DIVIDED DOSES WITH MEALS. ...GIVEN TO PT WITH HISTORY OF PEPTIC ULCER ONLY WITH GREATEST CAUTION & CAREFUL OBSERVATION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 864
...ABILITY OF DRUG TO DEPRESS HEMATOPOIESIS HAS BEEN DEMONSTRATED...PERIODIC BLOOD-CELL COUNTS ARE THEREFORE ADVISED DURING PROLONGED THERAPY. ADEQUATE PRECAUTIONS MUST ALSO BE TAKEN TO PREVENT INTRARENAL PPTN OF URATES...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 864
IN CLINICAL USE OF...URICOSURIC DRUGS, IT MUST BE KEPT IN MIND THAT THEY CAN ALTER PLASMA BINDING, DISTRIBUTION, & RENAL EXCRETION OF OTHER ORG ACIDS... WHETHER THESE BE NATURALLY OCCURRING...OR DRUGS & DRUG METABOLITES. /URICOSURICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 865
For more Drug Warnings (Complete) data for SULFINPYRAZONE (8 total), please visit the HSDB record page.
For the treatment of gout and gouty arthritis.
Sulfinpyrazone's pharmacologic activity is the potentiation of the urinary excretion of uric acid. It is useful for reducing the blood urate levels in patients with chronic tophaceous gout and acute intermittent gout, and for promoting the resorption of tophi.
Uricosuric Agents
Gout suppressants that act directly on the renal tubule to increase the excretion of uric acid, thus reducing its concentrations in plasma. (See all compounds classified as Uricosuric Agents.)
M - Musculo-skeletal system
M04 - Antigout preparations
M04A - Antigout preparations
M04AB - Preparations increasing uric acid excretion
M04AB02 - Sulfinpyrazone
...WELL ABSORBED AFTER ORAL ADMIN. ...BOUND TO PLASMA PROTEINS...98-99%. T/2... IN PLASMA AFTER IV INJECTION IS ABOUT 3 HR. AFTER ORAL ADMIN...URICOSURIC EFFECT MAY PERSIST FOR...10 HR. ALTHOUGH LITTLE...IS AVAILABLE FOR FILTRATION @ GLOMERULUS, IT IS SECRETED BY PROXIMAL TUBULE & UNDERGOES LITTLE PASSIVE BACK DIFFUSION...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 864
APPROX HALF OF ORALLY ADMIN DOSE APPEARS IN URINE WITHIN 24 HR. MOST OF DRUG (90%) IN URINE IS UNCHANGED; REMAINDER IS ELIMINATED AS...METABOLITE...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 864
...RAPIDLY EXCRETED IN MAN, TOGETHER WITH P-HYDROXYLATED METABOLITE. PLASMA T/2...IN RATS...16.2 HR, &...18% OF IP DOSE...WAS EXCRETED INTO URINE. PLASMA T/2 IN MAN...BETWEEN 1 & 3 HR. MAIN ROUTE OF ELIMINATION IN RAT...FECES (68% OF DOSE)...CONSIDERABLE PORTION...EXCRETED IN BILE WAS REABSORBED FROM INTESTINAL TRACT.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 578
...SULFINPYRAZONE...IS EXCRETED IN URINE OF RATS TO ONLY A SMALL EXTENT. ... CLEARED FROM CIRCULATION MAINLY THROUGH BILE...NO EVIDENCE OF ENTERO-HEPATIC CIRCULATION MECHANISM.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 133
UNCHANGED DRUG /IN RAT/ WAS PRINCIPAL EXCRETION PRODUCT & ONLY 28%...IN URINE & 24%...IN FECES & BILE WAS FOUND AS P-HYDROXYSULFINPYRAZOLE. FAILURE OF RAT TO EXCRETE A HIGH PROPORTION OF THE DRUG IN URINE MAY BE RELATED TO ITS LACK OF URICOSURIC PROPERTIES IN THIS SPECIES.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 578
MOST OF DRUG (90%) IN URINE IS UNCHANGED; REMAINDER IS ELIMINATED AS N1-P-HYDROXYPHENYL METABOLITE...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 864
.../IN RAT/ 28%...IN URINE & 24%...IN FECES & BILE WAS FOUND AS P-HYDROXYSULFINPYRAZOLE.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 578
Sulfinpyrazone has known human metabolites that include Sulfinpyrazone sulfone.
Sulfinpyrazone is a known human metabolite of sulfinpyrazone_sulfide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Approximately 4-6 hours
Sulfinpyrazone is an oral uricosuric agent (pyrazolone derivative) used to treat chronic or intermittent gouty arthritis. Sulfinpyrazone competitively inhibits the reabsorption of uric acid at the proximal convoluted tubule, thereby facilitating urinary excretion of uric acid and decreasing plasma urate concentrations. This is likely done through inhibition of the urate anion transporter (hURAT1) as well as the human organic anion transporter 4 (hOAT4). Sulfinpyrazone is not intended for the treatment of acute attacks because it lacks therapeutically useful analgesic and anti-inflammatory effects. Sulfinpyrazone and its sulfide metabolite possess COX inhibitory effects. Sulfinpyrazone has also been shown to be a UDP-glucuronsyltransferase inhibitor and a very potent CYP2C9 inhibitor. Sulfinpyrazone is also known to be a cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor as well as an inhibitor of several multridrug resistance proteins (MRPs).
...IN SUFFICIENT DOSAGE IS POTENT INHIBITOR OF RENAL TUBULAR REABSORPTION OF URIC ACID. ...SMALL DOSES MAY REDUCE EXCRETION...PRESUMABLY BY INHIBITING SECRETORY BUT NOT REABSORPTIVE TRANSPORT. BY COMPETITIVE INHIBITION...REDUCES RENAL TUBULAR SECRETION OF OTHER ORG ANIONS...AS PAH /PARA-AMINOHIPPURIC/ & SALICYLIC ACID.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 864