
1. Ammonium Salt Sulfisoxazole
2. Diolamine, Sulfisoxazole
3. Gantrisin
4. Gantrisin Pediatric
5. Monolithium Salt Sulfisoxazole
6. Monosodium Salt Sulfisoxazole
7. Neoxazoi
8. Pediatric, Gantrisin
9. Sulfadimethyloxazole
10. Sulfafurazol Fna
11. Sulfafurazole
12. Sulfasoxizole
13. Sulfisoxazole Diolamine
14. Sulfisoxazole, Ammonium Salt
15. Sulfisoxazole, Monolithium Salt
16. Sulfisoxazole, Monosodium Salt
17. Sulfisoxazole, Monosodium, Monomesylate Salt
18. Sulfisoxazole, Triammonium Salt
19. Tl Azole
20. Tl-azole
21. Triammonium Salt Sulfisoxazole
22. V Sul
23. V-sul
1. Sulfafurazole
2. 127-69-5
3. Sulphafurazole
4. Sulfisoxazol
5. Sulfafurazol
6. Sulfaisoxazole
7. Sulfofurazole
8. Sulfisoxasole
9. Gantrisin
10. Sulphaisoxazole
11. Sulfadimethylisoxazole
12. Alphazole
13. Amidoxal
14. Sulfalar
15. Sulfoxol
16. Sulfisoxazole Dialamine
17. Sulphafurazolum
18. Renosulfan
19. Sulfasoxazole
20. Sulfisonazole
21. Accuzole
22. Soxomide
23. Sulfazin
24. Sulfizin
25. Sulsoxin
26. Sosol
27. Roxosul Tablets
28. Sulphafurazol
29. Sulphisoxazol
30. Sulphofurazole
31. Chemouag
32. Cosoxazole
33. Gantrisona
34. Gantrosan
35. Isoxamin
36. Neazolin
37. Neoxazol
38. Sulfagan
39. Sulfasol
40. Uritrisin
41. Entusil
42. Entusul
43. Pancid
44. Soxisol
45. Stansin
46. Sulbio
47. Thiasin
48. Unisulf
49. Sulphadimethylisoxazole
50. 4-amino-n-(3,4-dimethylisoxazol-5-yl)benzenesulfonamide
51. Norilgan-s
52. Tl-azole
53. V-sul
54. Sk-soxazole
55. Sulfafurazolum
56. Sulphisoxazole
57. Dorsulfan
58. Gantrisine
59. Novazolo
60. Novosaxazole
61. Saxosozine
62. Sodizole
63. Soxamide
64. Soxazole
65. Soxitabs
66. Sulfapolar
67. Sulfisin
68. Sulfizol
69. Sulfizole
70. Urisoxin
71. Roxosul
72. Urogan
73. Vagilia
74. Azo Gantrisin
75. Dorsulfan Warthausen
76. Koro-sulf
77. Soxo
78. J-sul
79. 3,4-dimethyl-5-sulfonamidoisoxazole
80. 3,4-dimethyl-5-sulfanilamidoisoxazole
81. 5-sulfanilamido-3,4-dimethylisoxazole
82. Benzenesulfonamide, 4-amino-n-(3,4-dimethyl-5-isoxazolyl)-
83. 4-amino-n-(3,4-dimethyl-5-isoxazolyl)benzenesulfonamide
84. 3,4-dimethyl-5-sulphonamidoisoxazole
85. 3,4-dimethylisoxazole-5-sulfanilamide
86. G-sox
87. 3,4-dimethyl-5-sulphanilamidoisoxazole
88. 3,4-dimethylisoxazole-5-sulphanilamide
89. 5-sulphanilamido-3,4-dimethyl-isoxazole
90. 5-(4-aminophenylsulfonamido)-3,4-dimethylisoxazole
91. 5-(p-aminobenzenesulfonamido)-3,4-dimethylisoxazole
92. 5-(p-aminobenzenesulphonamido)-3,4-dimethylisoxazole
93. N1-(3,4-dimethyl-5-isoxazolyl)sulfanilamide
94. N'-(3,4)dimethylisoxazol-5-yl-sulphanilamide
95. Nci-c50022
96. Eryzole
97. Nu 445
98. Pediazole
99. U.s.-67
100. 4-amino-n-(3,4-dimethyl-5-isoxazolyl)benzenesulphonamide
101. 4-amino-n-(3,4-dimethyl-1,2-oxazol-5-yl)benzenesulfonamide
102. Sulfafurazole (inn)
103. Sulfafurazole [inn]
104. N(sup1)-(3,4-dimethyl-5-isoxazolyl)sulfanilamide
105. Chebi:102484
106. Gantrisin (tn)
107. Sulfisoxazolum
108. Component Of Azo-sulfizin
109. Mfcd00003150
110. Nsc-13120
111. Component Of Azo Gantrisin
112. Chembl453
113. Nsc-683536
114. N(sup 1)-(3,4-dimethyl-5-isoxazolyl)sulfanilamide
115. N(sup 1)-(3,4-dimethyl-5-isoxazolyl)sulphanilamide
116. 4-amino-n-(dimethyl-1,2-oxazol-5-yl)benzene-1-sulfonamide
117. Nsc13120
118. 3,4-dimethylisoxale-5-sulfanilamide
119. Nsc683536
120. 740t4c525w
121. Sulfazin (van)
122. Ncgc00016384-03
123. Ncgc00016384-10
124. Azosulfizin
125. Solfafurazolo
126. Sulphafuraz
127. 4-amino-n-(3,4-dimethyl-isoxazol-5-yl)-benzenesulfonamide
128. Astrazolo
129. Bactesulf
130. Barazae
131. Cas-127-69-5
132. Resoxol
133. Roxoxol
134. Sulfagen
135. Suloxsol
136. Ganda
137. 4-amino-n-(3,4-dimethyl-1,2-oxazol-5-yl)benzene-1-sulfonamide
138. Solfafurazolo [dcit]
139. Dsstox_cid_1292
140. N(1)-(3,4-dimethyl-5-isoxazolyl)sulfanilamide
141. Dsstox_rid_76064
142. Dsstox_gsid_21292
143. N(1)-(3,4-dimethyl-5-isoxazolyl)sulphanilamide
144. 5-(p-aminobenzenesulfonamido)-3,4-dimethylisooxale
145. Sulfafurazolum [inn-latin]
146. Wln: T5noj Cmswr Dz& D1 E1
147. Sulfanilamide,4-dimethyl-5-isoxazolyl)-
148. Smr000037657
149. Ccris 568
150. Hsdb 797
151. Benzenesulfonamide,4-dimethyl-5-isoxazolyl)-
152. Sr-01000003085
153. Einecs 204-858-9
154. Nsc 13120
155. Sulfisoxazole [usp:jan]
156. Nsc 683536
157. Brn 0263871
158. Gantrizin
159. Ai3-24003
160. Unii-740t4c525w
161. Component Of Azo Gantrisin Accuzole
162. Prestwick_726
163. Spectrum_001024
164. Tocris-0731
165. Prestwick0_000334
166. Prestwick1_000334
167. Prestwick2_000334
168. Prestwick3_000334
169. Spectrum2_001325
170. Spectrum3_001728
171. Spectrum4_000349
172. Spectrum5_001222
173. Azo-sulfizin (salt/mix)
174. Sulfisoxazole [mi]
175. Azo Gantrisin (salt/mix)
176. Epitope Id:122240
177. Sulfisoxazole [jan]
178. Sulfafurazole [iarc]
179. Sulfisoxazole (jp17/usp)
180. Sulfisoxazole [hsdb]
181. Sulfisoxazole, >=99.0%
182. Oprea1_680668
183. Oprea1_828173
184. Schembl23467
185. Sulfanilamide, N1-(3,4-dimethyl-5-isoxazolyl)-
186. Bspbio_000367
187. Bspbio_003376
188. Kbiogr_000757
189. Kbioss_001504
190. Sulfisoxazole [vandf]
191. 4-27-00-04747 (beilstein Handbook Reference)
192. Mls000028495
193. Mls000037737
194. Mls000563718
195. Bidd:gt0322
196. Divk1c_000579
197. Spectrum1500555
198. Sulfafurazole [mart.]
199. Spbio_001449
200. Spbio_002288
201. Sulfafurazole [who-dd]
202. Sulfisoxazole [usp-rs]
203. Bpbio1_000405
204. Dtxsid6021292
205. Sulfanilamide, N(sup 1)-(3,4-dimethyl-5-isoxazolyl)-
206. Hms501m21
207. Kbio1_000579
208. Kbio2_001504
209. Kbio2_004072
210. Kbio2_006640
211. Kbio3_002596
212. [(4-aminophenyl)sulfonyl](3,4-dimethylisoxazol-5-yl)amine
213. 4-amino-n-(3,4-dimethylisoxazol-5-yl)-benzenesulfonamide
214. Ninds_000579
215. Hms1569c09
216. Hms2092k13
217. Hms2096c09
218. Hms2233g23
219. Hms3259c06
220. Hms3266n17
221. Hms3374k11
222. Hms3411f03
223. Hms3655b03
224. Hms3675f03
225. Hms3713c09
226. Nu445
227. Pharmakon1600-01500555
228. Sulfisoxazole [green Book]
229. Sulfisoxazole [orange Book]
230. Albb-014131
231. Hy-b0323
232. Nsc33807
233. Nsc38588
234. Str04988
235. Sulfafurazole [ep Monograph]
236. Tox21_110409
237. Tox21_112958
238. Tox21_202265
239. Tox21_302851
240. Bdbm50034452
241. Ccg-39263
242. Nsc-33807
243. Nsc-38588
244. Nsc757343
245. S1916
246. Stk400452
247. Sulfisoxazole [usp Monograph]
248. Zinc96006009
249. Akos000119074
250. Akos000310021
251. Tox21_110409_1
252. Ac-1941
253. Db00263
254. Nc00536
255. Nsc-757343
256. Idi1_000579
257. Ncgc00016384-01
258. Ncgc00016384-02
259. Ncgc00016384-04
260. Ncgc00016384-05
261. Ncgc00016384-06
262. Ncgc00016384-07
263. Ncgc00016384-08
264. Ncgc00016384-09
265. Ncgc00016384-11
266. Ncgc00016384-12
267. Ncgc00016384-14
268. Ncgc00016384-15
269. Ncgc00023116-02
270. Ncgc00023116-05
271. Ncgc00023116-06
272. Ncgc00023116-07
273. Ncgc00023116-08
274. Ncgc00256605-01
275. Ncgc00259814-01
276. Sulfisoxazole 100 Microg/ml In Methanol
277. Sbi-0051529.p003
278. Azo Gantrisin Component Sulfisoxazole
279. Db-041869
280. Upcmld00x127-69-5:001
281. Ab00052104
282. Bb 0259593
283. Ft-0631743
284. Sw196872-3
285. U0098
286. Sulfisoxazole 1000 Microg/ml In Acetonitrile
287. C07318
288. D00450
289. H10712
290. N1-(3,4-dimethyl-5-isoxazolyl)sulphanilamide
291. Sulfisoxazole Component Of Azo Gantrisin
292. Ab00052104-14
293. Ab00052104_15
294. Ab00052104_16
295. Q372598
296. Sulfisoxazole, Vetranal(tm), Analytical Standard
297. 5-(4-aminophenylsulphonamido)-3,4-dimethylisoxazole
298. J-005528
299. Sr-01000003085-2
300. Sr-01000003085-5
301. Sr-01000003085-6
302. 5-(p-aminobenzenesulphonamide)-3,4-dimethylisoxazole
303. Brd-k50859149-001-05-4
304. Brd-k50859149-001-10-4
305. Sulfanilamide, N(sup1)-(3,4-dimethyl-5-isoxazolyl)-
306. Sulfanilamide, N.sup1.-(3,4-dimethyl-5-isoxazolyl)-
307. 4-amino-n-(3,4-dimethyl-5-isoxazolyl)benzenesulfonamid
308. F0848-0391
309. 4-amino-n-(3,4-dimethyl-5-isoxazolyl)benzene-sulfonamide
310. 4-amino-n-(3,4-dimethyl-5-isoxazolyl)benzenesulfonamide #
311. Sulfafurazole, European Pharmacopoeia (ep) Reference Standard
312. Sulfisoxazole, United States Pharmacopeia (usp) Reference Standard
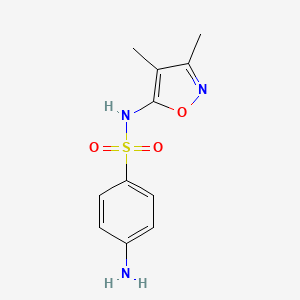
| Molecular Weight | 267.31 g/mol |
|---|---|
| Molecular Formula | C11H13N3O3S |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 267.06776246 g/mol |
| Monoisotopic Mass | 267.06776246 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 374 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents /SRP: Antibacterial/
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Acute, recurrent or chronic urinary tract infections (primarily pyelonephritis, pyelitis and cystitis) due to susceptible organisms (usually Escherichia coli, Klebsiella-Enterobacter, staphylococcus, Proteus mirabilis and, less frequently, Proteus vulgaris) in the absence of obstructive uropathy or foreign bodies. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Meningococcal meningitis where the organism has been demonstrated to be susceptible. Haemophilus influenzae meningitis as adjunctive therapy with parenteral streptomycin. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Meningococcal meningitis prophylaxis when sulfonamide-sensitive group A strains are known to prevail in family groups or larger closed populations. (The prophylactic usefulness of sulfonamides when group B or C infections are prevalent has not been proven and in closed population groups may be harmful.) Important Note: In vitro sulfonamide susceptibility tests are not always reliable. The test must be carefully coordinated with bacteriologic and clinical response. When the patient is already taking sulfonamides, follow-up cultures should have aminobenzoic acid added to the culture media. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
For more Therapeutic Uses (Complete) data for SULFISOXAZOLE (26 total), please visit the HSDB record page.
/Sulfisoxazole/ is contraindicated in the following patient populations: patients with a known hypersensitivity to sulfonamides; infants less than 2 months of age (except in the treatment of congenital toxoplasmosis as adjunctive therapy with pyrimethamine); pregnant women at term; and mothers nursing infants less than 2 months of age.
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Use in pregnant women at term, in infants less than 2 months of age and in mothers nursing infants less than 2 months of age is contraindicated because sulfonamides may promote kernicterus in the newborn by displacing bilirubin from plasma proteins.
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Fatalities associated with the administration of sulfonamides, although rare, have occurred due to severe reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia and other blood dyscrasias.
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Sulfonamides, including sulfisoxazole, should be discontinued at the first appearance of skin rash or any sign of an adverse reaction.
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
For more Drug Warnings (Complete) data for SULFISOXAZOLE (34 total), please visit the HSDB record page.
For the treatment of severe, repeated, or long-lasting urinary tract infections, meningococcal meningitis, acute otitis media, trachoma, inclusion conjunctivitis, nocardiosis, chancroid, toxoplasmosis, malaria and other bacterial infections.
Sulfisoxazole is a sulfonamide antibiotic. The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01EB - Short-acting sulfonamides
J01EB05 - Sulfafurazole
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AB - Sulfonamides
S01AB02 - Sulfafurazole
Route of Elimination
The mean urinary excretion recovery following oral administration of sulfisoxazole is 97% within 48 hours, of which 52% is intact drug, with the remaining as the N4-acetylated metabolite. It is excreted in human milk.
Following oral administration, sulfisoxazole is rapidly and completely absorbed; the small intestine is the major site of absorption, but some of the drug is absorbed from the stomach. Sulfonamides are present in the blood as free, conjugated (acetylated and possibly other forms) and protein-bound forms. The amount present as "free" drug is considered to be the therapeutically active form. Approximately 85% of a dose of sulfisoxazole is bound to plasma proteins, primarily to albumin; 65% to 72% of the unbound portion is in the nonacetylated form.
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Maximum plasma concentrations of intact sulfisoxazole following a single 2-g oral dose of sulfisoxazole to healthy adult volunteers ranged from 127 to 211 ug/mL (mean, 169 ug/mL) and the time of peak plasma concentration ranged from 1 to 4 hours (mean, 2.5 hours). ... After multiple-dose oral administration of 500 mg /four times a day/ to healthy volunteers, the average steady-state plasma concentrations of intact sulfisoxazole ranged from 49.9 to 88.8 ug/mL (mean, 63.4 ug/mL).
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Following a single 4-g dose of acetyl sulfisoxazole to healthy volunteers, maximum plasma concentrations of sulfisoxazole ranged from 122 to 282 ug/mL (mean, 181 ug/mL) for the pediatric suspension and occurred between 2 and 6 hours postadministration. /Acetyl-sulfisoxazole/
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Wide variation in blood levels may result following identical doses of a sulfonamide. Blood levels should be measured in patients receiving sulfonamides at the higher recommended doses or being treated for serious infections. Free sulfonamide blood levels of 50 to 150 ug/mL may be considered therapeutically effective for most infections, with blood levels of 120 to 150 ug/mL being optimal for serious infections. The maximum sulfonamide level should not exceed 200 ug/mL, since adverse reactions occur more frequently above this concentration.
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
For more Absorption, Distribution and Excretion (Complete) data for SULFISOXAZOLE (25 total), please visit the HSDB record page.
N1-acetyl sulfisoxazole is metabolized to sulfisoxazole by digestive enzymes in the gastrointestinal tract and is absorbed as sulfisoxazole. This enzymatic splitting is presumed to be responsible for slower absorption and lower peak blood concentrations than are attained following administration of an equal oral dose of sulfisoxazole. With continued administration of acetyl sulfisoxazole, blood concentrations approximate those of sulfisoxazole. /Acetyl-sulfisoxazole/
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Although the liver is the major site of metabolism, sulfonamides may also be metabolized in other body tissues. Most sulfonamides are metabolized mainly by N4-acetylation. The degree of acetylation, which is a function of time, varies from less than 5% for sulfamethizole to up to 40% for sulfadiazine. The N4-acetyl metabolites, which do not possess antibacterial activity, have greater affinity for plasma albumin than does the nonacetylated drug and are usually less soluble than the parent sulfonamide, particularly in acidic urine. Like acetyl derivatives, glucuronide derivatives do not possess antibacterial activity; however, glucuronide derivatives are water soluble, appear to resemble the nonacetylated sulfonamide in plasma binding capacity, and have not been associated with adverse effects. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
Identified urinary metabolites of sulfafurazole are acetylsulphisoxazole, sulphisoxazole-N-glucuronide, sulphisoxazole-N-sulphonate and sulphanilamide.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V24 280
In man it is excreted in urine as unchanged sulfisoxazole (56%), N(4)-acetyl derivative (18%), N(4)-glucuronide (3.4%), N(4)-sulfate (1.0%) & a second glucuronide which is probably N(2)-glucuronide of sulfisoxazole.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 181
Saturable metabolism of sulfisoxazole N1-acetyl in the rat during the initial pass of the drug from the intestinal lumen through the liver following oral administration of the drug (saturable first-pass metabolism) was investigated. The fraction of the total amount of drug recovered from the urine as the N4-conjugate fraction was apparent following the intravenous administration of sulfisoxazole acetyl or the oral administration of sulfisoxazole at the same dose levels.
PMID:1263077 Bloedow D, Hayton W; J Pharm Sci 65 (3): 334-8 (1976)
The elimination half-life of sulfisoxazole ranged from 4.6 to 7.8 hours after oral administration. The elimination of sulfisoxazole has been shown to be slower in elderly subjects (63 to 75 years) with diminished renal function (creatinine clearance, 37 to 68 mL/min).
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
The half-life of elimination from plasma ranged from 5.4 to 7.4 hr. /Acetyl-sulfisoxazole/
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
The half-life of sulfafurazole is approximately 6 hours.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V24 280
Sulfisoxazole is a competitive inhibitor of the enzyme dihydropteroate synthetase. It inhibits bacterial synthesis of dihydrofolic acid by preventing the condensation of the pteridine with para-aminobenzoic acid (PABA), a substrate of the enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid.
The sulfonamides are bacteriostatic agents and the spectrum of activity is similar for all. Sulfonamides inhibit bacterial synthesis of dihydrofolic acid by preventing the condensation of the pteridine with aminobenzoic acid through competitive inhibition of the enzyme dihydropteroate synthetase. Resistant strains have altered dihydropteroate synthetase with reduced affinity for sulfonamides or produce increased quantities of aminobenzoic acid.
US Natl Inst Health; DailyMed. Current Medication Information for Sulfisoxazole (March 2007). Available from, as of June 30, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
Sulfonamides are usually bacteriostatic in action. Sulfonamides interfere with the utilization of p-aminobenzoic acid (PABA) in the biosynthesis of tetrahydrofolic acid (the reduced form of folic acid) cofactors in susceptible bacteria. Sulfonamides are structural analogs of PABA and appear to interfere with PABA utilization by competitively inhibiting the enzyme dihydropteroate synthase, which catalyzes the formation of dihydropteroic acid (a precursor of tetrahydrofolic acid) from PABA and pteridine; however, other mechanism(s) affecting the biosynthetic pathway also may be involved. Compounds such as pyrimethamine and trimethoprim, which block later stages in the synthesis of folic acid, act synergistically with sulfonamides. Only microorganisms that synthesize their own folic acid are inhibited by sulfonamides; animal cells and bacteria which are capable of utilizing folic acid precursors or preformed folic acid are not affected by these drugs. The antibacterial activity of the sulfonamides is reportedly decreased in the presence of blood or purulent body exudates. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 424