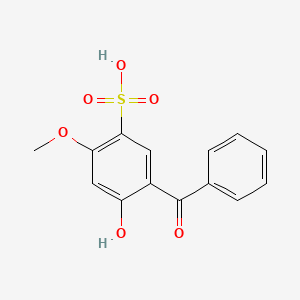

1. 2-hydroxy-4-methoxybenzophenone-5-sulfonic Acid
2. 5-benzoyl-4-hydroxy-2-methoxybenzene Sulfonic Acid
3. Benzophenone-4
4. Bp-4 Benzophenone
5. Sulisobenzone, Monosodium Salt
6. Uval
1. 4065-45-6
2. Benzophenone-4
3. 5-benzoyl-4-hydroxy-2-methoxybenzenesulfonic Acid
4. 2-hydroxy-4-methoxybenzophenone-5-sulfonic Acid
5. Sungard
6. Benzophenone 4
7. Uval
8. Seesorb 101s
9. Syntase 230
10. Uvinul Ms 40
11. Spectra-sorb Uv 284
12. Benzenesulfonic Acid, 5-benzoyl-4-hydroxy-2-methoxy-
13. Sulisobenzona
14. Sulisobenzonum
15. 2-hydroxy-4-methoxy-5-sulfobenzophenone
16. Ms 40
17. Uvinuc Ms 40
18. Sulfisobenzone
19. Uvinul Ms-40
20. Uvasorb S 5
21. Escalol 577
22. Viosorb 111
23. Uvinul D 5030
24. Nsc-60584
25. 1-phenol-4-sulfonic Acid, 2-benzoyl-5-methoxy-
26. 3-benzoyl-4-hydroxy-6-methoxybenzenesulfonic Acid
27. 2-benzoyl-5-methoxy-1-phenol-4-sulfonic Acid
28. 1w6l629b4k
29. Mfcd00024962
30. Nsc-760350
31. Uval Sodium Salt
32. Ncgc00159404-02
33. Ncgc00159404-04
34. Nsc 60584
35. Dsstox_cid_22436
36. Dsstox_rid_80023
37. Dsstox_gsid_42436
38. Uvinul Ms 40 Sodium Salt
39. Cyasorb Uv 284 Sodium Salt
40. Sulisobenzonum [inn-latin]
41. Sulisobenzona [inn-spanish]
42. Cas-4065-45-6
43. Nsc60584
44. Nsc654266
45. Hsdb 7422
46. Uvinul Ms-40 Substanz
47. 6628-37-1
48. Einecs 223-772-2
49. Brn 2889165
50. Unii-1w6l629b4k
51. Sulisobenzone [usan:usp:inn]
52. 5-benzoyl-4-hydroxy-2-methoxybenzene Sulfonic Acid
53. Sungard (tn)
54. Hmbs
55. Benzenesulfonic Acid, 5-benzoyl-4-hydroxy-2-methoxy-, Sodium Salt
56. Uval (*sodium Salt*)
57. Uvinul Ms40
58. Cbmicro_016354
59. Sulisobenzone (usp/inn)
60. Sulisobenzone [mi]
61. Ec 223-772-2
62. Sulisobenzone [inn]
63. 5-benzoyl-4-hydroxy-2-methoxybenzolsulfonsaeure
64. Spectra-sorb U.v. 284
65. Sulisobenzone [hsdb]
66. Sulisobenzone [usan]
67. Schembl16330
68. 2-hydroxy-4-methoxy-5-sulfonylbenzophenone(bp-4)
69. 5-benzoyl-4-hydroxy-2-methoxy-benzenesulfonic Acid
70. Mls004734662
71. Sulfisobenzone [vandf]
72. Sulisobenzone [mart.]
73. Benzophenone-4 [inci]
74. Sulisobenzone [usp-rs]
75. Sulisobenzone [who-dd]
76. Cyasorb Uv 284 (salt/mix)
77. Chembl2059073
78. Dtxsid2042436
79. 4-hydroxy-2-methoxy-5-(phenylcarbonyl)benzenesulfonic Acid
80. Chebi:135312
81. Sulisobenzone, Analytical Standard
82. Uvinul Ms 40 (*sodium Salt*)
83. Albb-025816
84. Cyasorb Uv 284 (*sodium Salt*)
85. Hy-b1162
86. Zinc1690324
87. Tox21_111639
88. Tox21_202332
89. Tox21_303469
90. S4652
91. Sulisobenzone [usp Monograph]
92. Akos015895600
93. Tox21_111639_1
94. Ccg-267557
95. Cs-4610
96. Db11185
97. Sulisobenzone (usan) (*sodium Salt*)
98. Ncgc00159404-03
99. Ncgc00159404-05
100. Ncgc00257488-01
101. Ncgc00259881-01
102. Ac-19869
103. As-12605
104. Smr001262265
105. Sy036839
106. 5-benzoyl-4-hydroxy-2-methoxy-besylic Acid
107. Db-049626
108. Ft-0612547
109. H0466
110. 2-benzoyl-5-methoxy-1-phenol-4-sulphonic Acid
111. D05964
112. F11240
113. A825218
114. J-509633
115. Q7636301
116. 5-benzoyl-4-hydroxy-2-methoxybenzenesulfonic Acid Ammoniate
117. 1-phenol-4-sulfonic Acid, 2-benzoyl-5-methoxy-, Sodium Salt
118. 4-hydroxy-2-methoxy-5-(oxo-phenylmethyl)benzenesulfonic Acid
119. Sulisobenzone, United States Pharmacopeia (usp) Reference Standard
120. 5-benzoyl-4-hydroxy-2-methoxybenzenesulfonic Acid, >=97.0% (hplc)
121. 5-benzoyl-4-hydroxy-2-methoxybenzenesulfonic Acid 100 Microg/ml In Methanol
| Molecular Weight | 308.31 g/mol |
|---|---|
| Molecular Formula | C14H12O6S |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 308.03545927 g/mol |
| Monoisotopic Mass | 308.03545927 g/mol |
| Topological Polar Surface Area | 109 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 462 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Daily use of a sunscreen with a high SPF (greater than 15) on usually exposed skin is recommended for residents of areas of high ... /solar radiation/ who work outdoors or ... /enjoy/ regular outdoor recreation. Daily use of a sunscreen can reduce the cumulative ... /solar/ exposure that causes actinic keratoses and squamous-cell carcinoma.
IARC Working Group on the Evaluation of Cancer-Preventive Agents (2001) Sunscreens (IARC Handbooks of Cancer Prevention, Vol. 5), Lyon, IARC; Unit of Chemoprevention: Cancer-Preventive Effects of Sunscreens.
Sunscreen agents are indicated for the prevention of sunburn. In addition to limiting the skin's exposure to the sun, using sunscreen agents regularly when in the sun may help reduce long-term sun damage such as premature aging of the skin and skin cancer. /Sunscreen agents, topical; Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
The manufacturers of sunscreen preparations with propellants warn that concentrating and subsequently inhaling the fumes from these preparations may be harmful or fatal. /Propellants/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Because the absorptive characteristics of skin of children younger than 6 months of age may differ from those of adults and because the immaturity of metabolic and excretory pathways of these children may limit their ability to eliminate any percutaneously absorbed sunscreen agent, sunscreen products should be used in children younger than 6 months of age only as directed by a clinician. It is possible that the characteristics of geriatric skin also differ from those of skin in younger adults, but these characteristics and the need for special considerations regarding use of sunscreen preparations in this age group are poorly understood. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Little information is available regarding the safety of chronic sunscreen usage, but commercially available physical and chemical sunscreens appear to have a low incidence of adverse effects. Derivatives of PABA, benzophenone, cinnamic acid, and salicylate and 2-phenylbenzimidazole-5-sulfonic acid have caused skin irritation including burning, stinging, pruritus, and erythema on rare occasions. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Sunscreens should not be used as a means of extending the duration of solar exposure, such as prolonging sunbathing, and should not be used as a substitute for clothing on usually unexposed sites, such as the trunk and buttocks. /Sunscreens/
IARC Working Group on the Evaluation of Cancer-Preventive Agents (2001) Sunscreens (IARC Handbooks of Cancer Prevention, Vol. 5), Lyon, IARC; Unit of Chemoprevention: Cancer-Preventive Effects of Sunscreens.
For more Drug Warnings (Complete) data for SULISOBENZONE (11 total), please visit the HSDB record page.
Sunscreening agents are used to prevent sunburn, actinic keratosis, and premature skin aging and to reduce the incidence of skin cancer.
Benzophenone sunscreens, applied topically, protect the skin from these harmful effects of ultraviolet light by chemically absorbing light energy (photons). Correct use of sunscreens serves to reduce the risk of sunburn. Sunscreen agents prevent the occurrence of squamous-cell carcinoma of the skin when used mainly during unintentional sun exposure. No conclusion can be drawn about the cancer-preventive activity of topical use of sunscreens against both basal-cell carcinoma and cutaneous melanoma. Use of sunscreens can extend the duration of intentional sun exposure, such as bathing in the sun.
Absorption
Does not penetrate the skin to a large degree, but enhances the ability of other chemicals to penetrate.
Route of Elimination
This drug's main metabolite is excreted in urine conjugated with glucuronic acid. No p-hydroxybenzohydrol was detected in urine or feces, in a study of pharmacokinetics in rats.
Solvents used in sunscreen products affect the stability and binding of the drug to the skin; in general, alcoholic solvents allow for the most rapid and deepest epidermal penetration of sunscreens. It appears that sunscreen agents are absorbed by the intact epidermis to varying degrees. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Benzophenone's main metabolic pathway in the rabbit is by reduction to benzhydrol. A small amount (1%) is converted to p-hydroxybenzophenone following oral administration to rats.
A surface coating of benzophenones decreases the amount of UV radiation absorbed by the skin by limiting the total amount of energy that reaches the skin. Benzophenone sunscreens, applied topically, protect the skin from these harmful effects of ultraviolet light by chemically absorbing light energy (photons). As this occurs, the benzophenone molecule becomes activated to higher energy levels. As the excited molecule returns to its ground state, the energy is released in the form of thermal energy. The hydroxyl group in the ortho position to the carbonyl group is believed to be a structural requirement for the benzophenones' absorption of UV light. This structural arrangement also contributes to the electronic stability of the molecule. Benzophenones absorb energy throughout the UV range, although the maximum UV absorbance is between 284 and 287 nm for the 2-hydroxybenzophenones.
Benzophenone sunscreens, applied topically, protect the skin from these harmful effects of ultraviolet light by chemically absorbing light energy (photons). As this occurs, the Benzophenone molecule becomes excited to higher energy levels. As the excited molecule returns to its ground state, the energy is released in the form of thermal energy. The hydroxyl group in the ortho position to the carbonyl group is believed to be a structural requirement for the Benzophenones' absorption of UV light. This structural arrangement also contributes to the electronic stability of the molecule. Thus, a surface coating of Benzophenones decreases the amount of UV radiation absorbed by the skin by limiting the total amount of energy that reaches the skin. Benzophenones absorb energy throughout the UV range, though maximum absorbance is between 284 and 287 nm for the 2-hydroxybenzophenones ...
Cosmetic Ingredient Review; Final Report on the Safety Assessment of Benzophenones-1, -3, -4, -5, -9, and -11; Journal of the American College of Toxicology 2 (5): 35-78 (1983). Available form, as of September 12, 2013: https://online.personalcarecouncil.org/ctfa-static/online/lists/cir-pdfs/pr219.pdf
Diminish the penetration of ultraviolet (UV) light through the epidermis by absorbing UV radiation within a specific wavelength range. The amount and wavelength of UV radiation absorbed are affected by the molecular structure of the sunscreen agent. /Sunscreen agents, topical/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.