

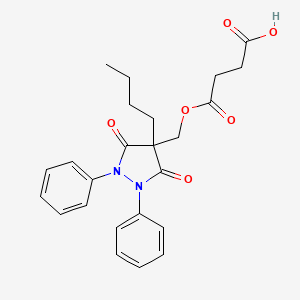

1. 1,2-diphenyl-4-n-butyl-4-hydroxymethyl-3,5-dioxopyrazolidine Hemisuccinate
2. 4-butyl-4-(beta-carboxypropionyloxymethyl)-1,2-diphenyl-3,5-pyrazolidinedione
1. 27470-51-5
2. Calibene
3. Suxibuzona
4. Suxibuzonum
5. Solurol
6. Flogos
7. 4-hydroxymethylbutazolidine Hemisuccinate
8. 4-((4-butyl-3,5-dioxo-1,2-diphenylpyrazolidin-4-yl)methoxy)-4-oxobutanoic Acid
9. Ae-17
10. 4-butyl-4-hydroxymethyl-1,2-diphenyl-3,5-pyrazolidinedione Hydrogen Succinate
11. 4-[(4-butyl-3,5-dioxo-1,2-diphenylpyrazolidin-4-yl)methoxy]-4-oxobutanoic Acid
12. Chebi:32173
13. Nsc-757866
14. 86tdz5wp2b
15. 4-butyl-4-(hydroxymethyl)-1,2-diphenyl-3,5-pyrazolidinedione Hydrogen Succinate (ester)
16. Succinic Acid, Monoester With 4-butyl-4-(hydroxymethyl)-1,2-diphenyl-3,5-pyrazolidinedione
17. Ncgc00016799-01
18. Aflogan
19. Flamilon
20. Alfide
21. Cas-27470-51-5
22. Dsstox_cid_1296
23. 3,5-pyrazolidinedione, 4-butyl-4-(hydroxymethyl)-1,2-diphenyl-, Hydrogen Succinate (ester)
24. Dsstox_rid_76066
25. Dsstox_gsid_21296
26. Suxibuzonum [inn-latin]
27. Suxibuzona [inn-spanish]
28. Butanedioic Acid, Mono((4-butyl-3,5-dioxo-1,2-diphenyl-4-pyrazolidinyl)methyl) Ester
29. Butanedioic Acid, Mono[(4-butyl-3,5-dioxo-1,2-diphenyl-4-pyrazolidinyl)methyl] Ester
30. 4-butyl-4-[hydroxymethyl]-1,2-diphenyl-3,5-pyrazolidinedione Hydrogen Succinate [ester]
31. Ae 17
32. Sr-01000841241
33. Einecs 248-477-6
34. Unii-86tdz5wp2b
35. Brn 0904563
36. Suxibuzon
37. Suxibuzone [inn:ban:jan]
38. Prestwick_113
39. Spectrum_000321
40. Suxibuzone (jan/inn)
41. Suxibuzone [inn]
42. Suxibuzone [jan]
43. Prestwick0_000658
44. Prestwick1_000658
45. Prestwick2_000658
46. Prestwick3_000658
47. Spectrum2_000966
48. Spectrum3_000664
49. Spectrum4_000433
50. Spectrum5_001113
51. Epitope Id:124941
52. 4-butyl-4-(beta-carboxypropionyl-oxymethyl)-1,2-diphenyl-3,5-pyrazolidinedione
53. Suxibuzone [mart.]
54. 3-((4-butyl-3,5-dioxo-1,2-diphenyl-4-pyrazolidinyl)methoxycarbonyl)propionsaeure
55. Suxibuzone [who-dd]
56. Schembl25810
57. Bspbio_000695
58. Bspbio_002327
59. Kbiogr_000786
60. Kbioss_000801
61. Mls002153938
62. Divk1c_000171
63. Spectrum1501157
64. Spbio_000971
65. Spbio_002616
66. Bpbio1_000765
67. Chembl1414320
68. Dtxsid6021296
69. Suxibuzone [ep Impurity]
70. Hms500i13
71. Kbio1_000171
72. Kbio2_000801
73. Kbio2_003369
74. Kbio2_005937
75. Kbio3_001547
76. Brd5826
77. Suxibuzone [ep Monograph]
78. Ninds_000171
79. Hms1570c17
80. Hms1923i17
81. Hms2097c17
82. Hms2232e17
83. Hms3371a12
84. Hms3714c17
85. Pharmakon1600-01501157
86. Brd-5826
87. Hy-b1079
88. Zinc3875039
89. Tox21_110615
90. Tox21_202352
91. Tox21_302778
92. Ccg-40180
93. Nsc757866
94. Akos024374988
95. Tox21_110615_1
96. Cs-4635
97. Db13232
98. Nsc 757866
99. 4-[(4-butyl-3,5-dioxo-1,2-diphenyl-4-pyrazolidinyl)methoxy]-4-oxobutanoic Acid
100. 4-{[(4-butyl-3,5-dioxo-1,2-diphenylpyrazolidin-4-yl)methyl]oxy}-4-oxobutanoic Acid
101. 4-butyl-4-[hydroxymethyl]-1,2-diphenyl-3,5-pyrazolidinedionehydrogensuccinate[ester]
102. Idi1_000171
103. Ncgc00016799-02
104. Ncgc00016799-03
105. Ncgc00016799-04
106. Ncgc00016799-05
107. Ncgc00016799-06
108. Ncgc00016799-08
109. Ncgc00095261-01
110. Ncgc00095261-02
111. Ncgc00095261-03
112. Ncgc00095261-04
113. Ncgc00256470-01
114. Ncgc00259901-01
115. Smr001233279
116. Sbi-0051667.p002
117. Db-047228
118. Ab00052229
119. Ft-0630533
120. D01289
121. Ab00052229_08
122. Ab00052229_09
123. 470s515
124. A936730
125. J-016775
126. Q7650623
127. Sr-01000841241-2
128. Sr-01000841241-3
129. Brd-k78815826-001-05-4
130. Brd-k78815826-001-08-8
131. Suxibuzone, European Pharmacopoeia (ep) Reference Standard
132. 4-butyl-4-(.beta.-carboxypropionyl-oxymethyl)-1,2-diphenyl-3,5-pyrazolidinedione
| Molecular Weight | 438.5 g/mol |
|---|---|
| Molecular Formula | C24H26N2O6 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 11 |
| Exact Mass | 438.17908655 g/mol |
| Monoisotopic Mass | 438.17908655 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 661 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA22 - Suxibuzone
