

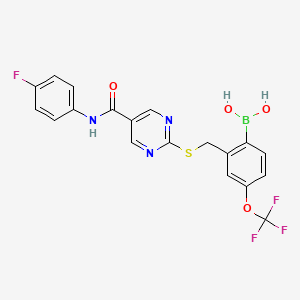

1. (2-((5-((4-fluorophenyl)carbamoyl)pyrimidin-2-yl)sulfanylmethyl)-4-(trifluoromethoxy)phenyl)boronic Acid
2. Incb057643
1. Sx-682
2. 1648843-04-2
3. Unii-h5212r2dpm
4. H5212r2dpm
5. (2-(((5-((4-fluorophenyl)carbamoyl)pyrimidin-2-yl)thio)methyl)-4-(trifluoromethoxy)phenyl)boronic Acid
6. [2-[[5-[(4-fluorophenyl)carbamoyl]pyrimidin-2-yl]sulfanylmethyl]-4-(trifluoromethoxy)phenyl]boronic Acid
7. (2-(((5-((4-fluorophenyl)carbamoyl)pyrimidin-2-yl)thio)methyl)-4-(trifluoromethoxy)phenyl)boronicacid
8. B-(2-(((5-(((4-fluorophenyl)amino)carbonyl)-2-pyrimidinyl)thio)methyl)-4-(trifluoromethoxy)phenyl)boronic Acid
9. Boronic Acid, B-(2-(((5-(((4-fluorophenyl)amino)carbonyl)-2-pyrimidinyl)thio)methyl)-4-(trifluoromethoxy)phenyl)-
10. Chembl4297480
11. Gtpl10165
12. Sx682
13. Bcp32154
14. Ex-a4295
15. Mfcd28502254
16. S8947
17. Sx 682; Sx682
18. Sb17394
19. Ac-36549
20. Hy-119339
21. Cs-0067128
22. D81536
| Molecular Weight | 467.2 g/mol |
|---|---|
| Molecular Formula | C19H14BF4N3O4S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 7 |
| Exact Mass | 467.0734199 g/mol |
| Monoisotopic Mass | 467.0734199 g/mol |
| Topological Polar Surface Area | 130 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)