


1. Fx 1006a
2. Fx-1006a
3. Fx1006a
4. Tafamidis Meglumine
5. Vyndamax
6. Vyndaqel
1. 594839-88-0
2. Vyndamax
3. Fx-1006
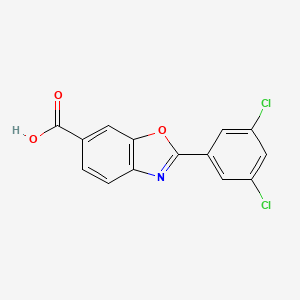
4. 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic Acid
5. 2-(3,5-dichlorophenyl)-6-benzoxazole Carboxylic Acid
6. 8fg9h9d31j
7. Chebi:78538
8. 2-(3,5-dichlorophenyl)benzoxazole-6-carboxylic Acid
9. 2-(3,5-dichlorophenyl)benzo[d]oxazole-6-carboxylic Acid
10. 594839-88-0 (free Acid)
11. Fx1006
12. Mfcd16621109
13. Fx-1005
14. 6-benzoxazolecarboxylic Acid, 2-(3,5-dichlorophenyl)-
15. Tafamidis [usan]
16. Tafamidis [usan:inn]
17. Tafamidisum
18. Unii-8fg9h9d31j
19. 4his
20. Tafamidis-meglumine
21. Vyndamax (tn)
22. 3mi
23. Tafamidis [inn]
24. Tafamidis [jan]
25. Tafamidis [mi]
26. Tafamidis [mart.]
27. Tafamidis [who-dd]
28. Tafamidis (jan/usan/inn)
29. Schembl442508
30. Gtpl8378
31. Tafamidis [orange Book]
32. Chembl2103837
33. Pf-06291826(tafamidis)
34. Dtxsid00208185
35. Hms3741e09
36. Bcp29089
37. Ex-a3575
38. Bdbm50197883
39. S6465
40. Zinc43206271
41. Akos017550076
42. Db11644
43. Vs-0125
44. Ncgc00390731-01
45. 137464-18-7
46. Hy-14852
47. Sy217402
48. Db-072645
49. Ft-0674793
50. A14111
51. C75776
52. D09673
53. 839d880
54. A869196
55. Q519447
56. 2-(3,5-dichloro-phenyl)-benzooxazole-6-carboxylic Acid
57. Z2588039578
58. Discontinued Until Pfizer Approves It For Our Agreement""
| Molecular Weight | 308.1 g/mol |
|---|---|
| Molecular Formula | C14H7Cl2NO3 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 306.9802985 g/mol |
| Monoisotopic Mass | 306.9802985 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 371 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tafamidis is indicated to treat cardiomyopathy of wild type or hereditary transthyretin-mediated amyloidosis in adults.
FDA Label
Tafamidis stabilizes transthyretin tetramers, reducing the amount of monomers available for amyloidogenesis. It has a long duration of action as it is given once daily, and a wide therapeutic window.
N07XX08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N07 - Other nervous system drugs
N07X - Other nervous system drugs
N07XX - Other nervous system drugs
N07XX08 - Tafamidis
Absorption
Tafamidis reaches a Cmax of 1430.93ng/mL with a Tmax of 1.75h fasted and 4h fed. The AUC of tafamidis is 47,864.31ng\*h/mL.
Route of Elimination
A 20mg oral dose of tafamidis is approximately 59% recovered in the feces, largely as unchanged drug. Approximately 22% of a 20mg oral dose is recovered in the urine, mostly as the glucuronide metabolite.
Volume of Distribution
The apparent volume of distribution at steady state is 18.5L.
Clearance
The oral clearance of tafamidis is 0.263L/h. The apparent total clearance is 0.44L/h.
Tafamidis is largely not subject to first pass or oxidative metabolism, being 90% unchanged after in in vitro experiments. Preclinical data suggest tafamidis is mainly metabolized through glucuronidation and excreted in bile.
The half life of tafamidis is 49h.
Genetic mutations or natural misfolding of transthyretin destabalizes transthyretin tetramers, leading to their dissociation and aggregation in tissues, and disrupting the normal function of these tissues. Tafamidis binds to transthyretin tetramers at the thyroxin binding sites, stabilizing the tetramer, reducing the availability of monomers for amyloidogenesis.
