

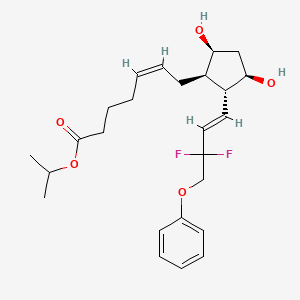

1. 1-methylethyl (5z)-7-((1r,2r, 3r,5s)-2-((1e)-3,3-difluoro-4-phenoxy -1-butenyl)-3,5-dihydroxycyclopentyl)-5-heptenoate
2. Afp-168
1. 209860-87-7
2. Afp-168
3. Taflotan
4. Zioptan
5. Saflutan
6. Mk-2452
7. 1o6wq6t7g3
8. Chebi:66899
9. Mk2452
10. (z)-isopropyl 7-((1r,2r,3r,5s)-2-((e)-3,3-difluoro-4-phenoxybut-1-en-1-yl)-3,5-dihydroxycyclopentyl)hept-5-enoate
11. Tapros
12. Isopropyl (5z)-7-{(1r,2r,3r,5s)-2-[(1e)-3,3-difluoro-4-phenoxybut-1-en-1-yl]-3,5-dihydroxycyclopentyl}hept-5-enoate
13. Propan-2-yl (5z)-7-[(1r,2r,3r,5s)-2-[(1e)-3,3-difluoro-4-phenoxybut-1-en-1-yl]-3,5-dihydroxycyclopentyl]hept-5-enoate
14. Propan-2-yl (z)-7-[(1r,2r,3r,5s)-2-[(e)-3,3-difluoro-4-phenoxybut-1-enyl]-3,5-dihydroxycyclopentyl]hept-5-enoate
15. Zioptan (tn)
16. Isopropyl (5z)-7-((1r,2r,3r,5s)-2-((1e)-3,3-difluoro-4-phenoxybut-1-enyl)-3,5-dihydroxycyclopentyl)hept-5-enoate
17. Isopropyl (5z)-7-{(1r,2r,3r,5s)-2-((1e)-3,3-difluoro-4-phenoxybut-1-enyl)-3,5-dihydroxycyclopentyl}hept-5-enoate
18. Tafluprost [inn]
19. Tafluprost [mi]
20. Tafluprost [jan]
21. Tafluprost [inci]
22. Tafluprost [usan]
23. Tafluprost [vandf]
24. Tafluprost [mart.]
25. Tafluprost [who-dd]
26. Unii-1o6wq6t7g3
27. Tafluprost (jan/usan/inn)
28. Tafluprost [usan:inn:ban]
29. Gtpl7451
30. Schembl1286148
31. Chembl1963683
32. Tafluprost [orange Book]
33. Ex-a564
34. Dtxsid401021504
35. Hms3649f04
36. Mk2452afp-168
37. Hy-b0600
38. De-085
39. De-118
40. S4851
41. Zinc13912394
42. Akos025294885
43. Ccg-269257
44. Db08819
45. As-75193
46. D06274
47. 860t877
48. Sr-01000946707
49. J-502635
50. Q2139543
51. Sr-01000946707-1
52. (z)-isopropyl 7-((1r,2r,3r,5s)-2-((e)-3,3-difluoro-4-phenoxybut-1-en-1-yl)-3,5-dihydroxy Cyclopentyl)hept-5-enoate
53. (z)-isopropyl7-((1r,2r,3r,5s)-2-((e)-3,3-difluoro-4-phenoxybut-1-en-1-yl)-3,5-dihydroxycyclopentyl)hept-5-enoate
54. 1-methylethyl (5-z)-7-[(1r,2r,3r,5s)-2-[(1e)-3,3-difluoro-4-phenoxy-1-buten-1-yl]-3,5-dihydroxycyclopentyl]-5-heptenoate
55. 1-methylethyl (5z)-7-((1r,2r, 3r,5s)-2-((1e)-3,3-difluoro-4-phenoxy -1-butenyl-3,5-dihydroxycyclopentyl)-5-heptenoate
56. 1-methylethyl (5z)-7-{(1r,2r,3r,5s)-2-((1e)-3,3-difluoro-4-phenoxybut-1-enyl)-3,5- Dihydroxycyclopentyl}hept-5-enoate
57. 5-heptenoic Acid, 7-((1r,2r,3r,5s)-2-((1e)-3,3-difluoro-4-phenoxy-1-buten-1-yl)-3,5- Dihydroxycyclopentyl)-, 1-methylethyl Ester, (5z)-
58. 5-heptenoic Acid, 7-[(1r,2r,3r,5s)-2-[(1e)-3,3-difluoro-4-phenoxy-1-buten-1-yl]-3,5-dihydroxycyclopentyl]-, 1-methylethyl Ester, (5z)-
59. 5-heptenoic Acid, 7-[(1r,2r,3r,5s)-2-[(1e)-3,3-difluoro-4-phenoxy-1-butenyl]-3,5-dihydroxycyclopentyl]-, 1-methylethyl Ester, (5z)-
60. Propan-2-yl (z)-7-[(1r,2r,3r,5s)-2-[(e)-3,3-difluoro-4-(phenoxy)but-1-enyl]-3,5-dihydroxycyclopentyl]hept-5-enoate
| Molecular Weight | 452.5 g/mol |
|---|---|
| Molecular Formula | C25H34F2O5 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 13 |
| Exact Mass | 452.23743050 g/mol |
| Monoisotopic Mass | 452.23743050 g/mol |
| Topological Polar Surface Area | 76 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 614 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Zioptan |
| PubMed Health | Tafluprost (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | Tafluprost is a fluorinated analog of prostaglandin F2. The chemical name for tafluprost is 1-methylethyl (5Z)-7-{(1R, 2R, 3R, 5S)-2-[(1E)-3,3-difluoro-4-phenoxy-1-butenyl}-3,5-dihydroxycyclopentyl]-5-heptenoate. The molecular formula of tafluprost... |
| Active Ingredient | Tafluprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.0015% |
| Market Status | Prescription |
| Company | Oak Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Zioptan |
| PubMed Health | Tafluprost (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | Tafluprost is a fluorinated analog of prostaglandin F2. The chemical name for tafluprost is 1-methylethyl (5Z)-7-{(1R, 2R, 3R, 5S)-2-[(1E)-3,3-difluoro-4-phenoxy-1-butenyl}-3,5-dihydroxycyclopentyl]-5-heptenoate. The molecular formula of tafluprost... |
| Active Ingredient | Tafluprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.0015% |
| Market Status | Prescription |
| Company | Oak Pharms |
Tafluprost is indicated for reducing elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
FDA Label
Treatment of glaucoma
Tafluprost is a novel prostaglandin analog with a high affinity for the fluoroprostaglandin (FP) receptor PGF2. Tafluprost has an affinity for the FP receptor that is approximately 12 times higher than that of the carboxylic acid of latanoprost, but with almost no potential to bind to other receptors.
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EE - Prostaglandin analogues
S01EE05 - Tafluprost
Absorption
Following instillation, tafluprost is absorbed through the cornea and is hydrolyzed to the biologically active acid metabolite, tafluprost acid. Tafluprost is an ester which makes the drug lipophillic enough to be quickly absorbed through. When administered to the eye, the peak plasma concentration (Cmax) and time to peak plasma concentration (Tmax) of tafluprost acid in healthy subjects was 26 pg/mL and 10 minutes respectively. a AUC, tafluprost acid = 394 pg*min/mL - 432 pg*min/mL.
Route of Elimination
Mean plasma tafluprost acid concentrations were below the limit of quantification of the bioanalytical assay (10 pg/mL) at 30 minutes following topical ocular administration of tafluprost 0.0015% ophthalmic solution. In male rats, it was observed that tafluprost was excreted into the feces.
Volume of Distribution
The highest concentration of tafluprost acid was found in the cornea and conjunctiva.
Tafluprost is an ester prodrug which is rapidly hydrolyzed by corneal esterases to form its biologically active acid metabolite. Tafluprost acid is further metabolized via fatty acid -oxidation and phase II conjugation into 1,2,3,4-tetranor acid.
Tafluprost acid is a prostanoid selective FP receptor agonist that is believed to reduce the intraocular pressure (IOP) by increasing the outflow of aqueous humor. Studies in animals and humans suggest that the main mechanism of action is increased uveoscleral outflow.

