

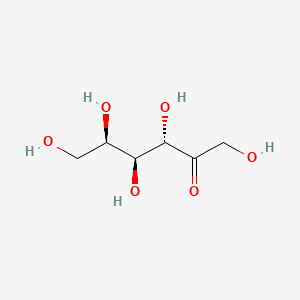

1. D-tagatose
2. Tagatose
3. Tagatose, (alpha-d)-isomer
4. Tagatose, (beta-d)-isomer
5. Tagatose, (d)-isomer
6. Tagatose, (dl)-isomer
1. 87-81-0
2. D-lyxo-hexulose
3. Lyxo-2-hexulose
4. 17598-81-1
5. Tagatose, D-
6. Naturlose
7. Keto-d-tagatose
8. Tagatose [nf]
9. Chebi:47693
10. T7a20y888y
11. Tagatose (nf)
12. Rel-(3s,4s,5r)-1,3,4,5,6-pentahydroxyhexan-2-one
13. Dl-tagatose
14. Einecs 201-772-3
15. Unii-t7a20y888y
16. Mfcd00134449
17. D-tagatose [mi]
18. D-tagatose [fcc]
19. Tagatose [mart.]
20. Tagatose [usp-rs]
21. Schembl4035
22. Tagatose, D- [ii]
23. Chembl1236183
24. Zinc2516866
25. D-(-)-tagatose, >=98.5%
26. S5387
27. D-(-)-tagatose, Analytical Standard
28. Akos015856492
29. Akos015902493
30. Ccg-266424
31. Db04936
32. Ncgc00248704-01
33. As-44378
34. Hy-42680
35. Cs-0028435
36. T1501
37. D09007
38. Wurcs=2.0/1,1,0/[ho112h]/1/
39. 134t449
40. Q414089
41. W-200517
42. Tagatose, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 180.16 g/mol |
|---|---|
| Molecular Formula | C6H12O6 |
| XLogP3 | -3.2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 180.06338810 g/mol |
| Monoisotopic Mass | 180.06338810 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 147 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Intended for use as a therapeutic adjunct in the treatment of type II diabetes.
Oral tagatose significantly blunts the rise in plasma glucose seen after oral glucose in patients with diabetes mellitus in a dose-dependent manner without significantly affecting insulin levels. The minimal elevation of plasma tagatose levels in normal patients and the adverse gastrointestinal effects seen following larger doses of tagatose support poor absorption of this hexose and suggest that tagatose may act by attenuating glucose absorption in the intestine.
Iron Chelating Agents
Organic chemicals that form two or more coordination links with an iron ion. Once coordination has occurred, the complex formed is called a chelate. The iron-binding porphyrin group of hemoglobin is an example of a metal chelate found in biological systems. (See all compounds classified as Iron Chelating Agents.)
Absorption
Only 15-20 percent of tagatose is absorbed in the small intestine.
Tagatose does not influence the level of blood glucose nor insulin levels.
he steps in the metabolism of tagatose are identical to those for fructose or fruit sugar but tagatose is incompletely absorbed. Only 15-20 percent of tagatose is absorbed in the small intestine. The major part of ingested tagatose is fermented in the colon by indigenous microflora, resulting in the production of short-chain fatty acids. The short chain fatty acids are absorbed almost completely and metabolized.