

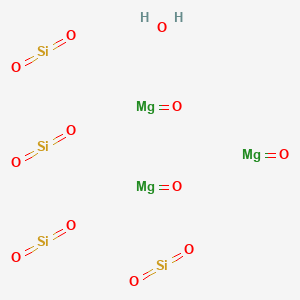

1. Talc
2. Talcum Powder
1. 14807-96-6
2. Pulvistalci
3. Magnesium Silicate, Hydrous
4. 13376-74-4
5. Magnesium Silicate Monohydrate (talc)
6. Mfcd00792903
7. 78229-94-4
8. Purified Talc
9. Talc Nanoparticles
10. Talc, Cp
11. Talc, -350 Mesh
12. Talc, Powder, 10 Mum
13. Magnesiumsilicatemonohydrate325m
14. Talc, Vetec(tm) Reagent Grade
15. Chembl3989756
16. Magnesium Silicate Xhydrate 800m
17. Talc, Tested According To Ph.eur.
18. Magnesium Silicate Monohydrate 325m
19. Ft-0645101
20. Ab01568263_01
21. Talc, Meets Analytical Specification Of Ph.??eur., Bp, Powder
22. 149925-00-8
| Molecular Weight | 379.27 g/mol |
|---|---|
| Molecular Formula | H2Mg3O12Si4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 0 |
| Exact Mass | 377.8174567 g/mol |
| Monoisotopic Mass | 377.8174567 g/mol |
| Topological Polar Surface Area | 189 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 20.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 8 |
Talc intrapleural aerosol is indicated to prevent recurrence of malignant pleural effusions in symptomatic patients. It is administered during thoracoscopy or open thoracotomy.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
...Talc insufflation, either as a poudrage or by using slurry, is recognized as an effective treatment /for spontaneous pneumothorax/.
PMID:14602332 Milton R, Cale AR; Ann Thorac Surg 76 (5): 1740-1 (2003)
...The case of a 78 yr-old man /is presented,/ who was treated with talc pleurodesis for recurrent pleural effusion and subsequently developed a massive hemothorax, a rare complication.
PMID:15384468 Hirota B et al; Hawaii Med J 63 (7): 208-10 (2004)
/The case of/ ...a young woman with a large, calcified anterior mediastinal mass discovered 18 months following a left talc pleurodesis /is reported/.
PMID:12607675 Ahmed Z, Shrager JB; Ann Thorac Surg 75 (2): 568-9 (2003)
Antiperspirants
Agents that are put on the SKIN to reduce SWEATING or prevent excess sweating (HYPERHIDROSIS). (See all compounds classified as Antiperspirants.)
Foreign body granulomas containing talc fibers are to be found in lung, pleura, diaphragm, pericardium, and gastric wall ... .
Hamilton, A., and H. L. Hardy. Industrial Toxicology. 3rd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1974., p. 441
...Transmission electron microscopy and energy dispersive X-ray spectroscopy /was used to analyze/ the total fibrous and nonfibrous mineral content of the lungs of a series of 14 male smokers with lung cancer but with no history of occupational dust exposure, and of a series of 14 control men matched by age, smoking history and general occupational class. The average concentration of mineral fibers and nonfiberous particles were 3.8 and 2.0 times higher in the group with cancer. Kaolinite, talc, mica, feldspars and crystalline silica comprised the majority of fibrous and nonfibrous particles in both groups.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V42 208 (1987)
Mice that received a sterile sc injection of talc were studied by measuring the incorporation of radioactive leucine and glucosamine into liver and plasma protein and the talc gramuloma at various intervals between 2 and 528 hours after injection. Incorporation into plasma proteins indicated a biphasic response with a marked increased incorporation into the perchloric acid insoluble fraction at 21 hours, a return to normal values at 45 hours, and a similar marked increase into the perchloric acid soluble fraction at 45 hours with a gradual return toward normal values. The response was dependent upon the amount of talc injected.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V1 526
Using radioactive tracer techniques in rats, mice, guinea pigs, and hamsters, no intestinal absorption or translocation of ingested talc to the liver and kidneys was detected.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V1 527
For more Absorption, Distribution and Excretion (Complete) data for TALC (7 total), please visit the HSDB record page.
In hamsters, ...the biological half-life of talc deposited in the alveoli was 7-10 days, and the alveolar clearance was basically complete 4 months after exposure.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V1 526