

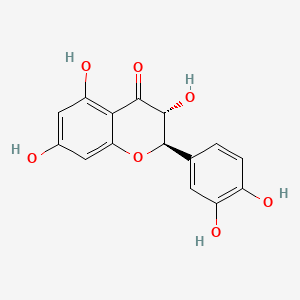

1. Dihydroquercetin
1. (+)-taxifolin
2. 480-18-2
3. Dihydroquercetin
4. Distylin
5. Taxifoliol
6. (2r,3r)-dihydroquercetin
7. 24198-97-8
8. (+)-dihydroquercetin
9. Lavitol
10. (+/-)-taxifolin
11. Taxifolin-(+)
12. 17654-26-1
13. Diquertin
14. Lariksin
15. 2,3-dihydroquercetin
16. Flamena D
17. (2r,3r)-3,3',4',5,7-pentahydroxyflavanone
18. (2r,3r)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one
19. (2r,3r)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychroman-4-one
20. Taxifolin (dihydroquercetin)
21. 3,5,7,3',4'-pentahydroxyflavanone
22. (2r,3r)-(+)-taxifolin
23. 4h-1-benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-, (2r,3r)-
24. (+/-)-dihydroquercetin
25. Taxifolin, (+/-)-
26. (2r,3r)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4h-chromen-4-one
27. Eas93sc1vs
28. Dihydroquercetin, (+/-)-
29. (2r-trans)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-4-benzopyrone
30. Flavanone, 3,3',4',5,7-pentahydroxy-
31. Chebi:17948
32. (+)-(2r,3r)-dihydroquercetin
33. 9sob9e3987
34. (?)-taxifolin
35. (2r,3r)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chroman-4-one
36. Rel-(2r,3r)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychroman-4-one
37. (2r,3r)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-4h-1-benzopyran-4-one
38. 4h-1-benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-, (2r-trans)-
39. 4h-1-benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-, Trans-(+/-)-
40. Dqh
41. Smr000466389
42. Trans-dihydroquercetin
43. (+)-trans-taxifolin
44. 4h-1-benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-
45. (+/-)-taxifolin Hydrate
46. Unii-9sob9e3987
47. Ccris 9292
48. Taxifolin (tax)
49. Einecs 207-543-4
50. Mfcd00006845
51. Flamena
52. (+)-trans Taxifolin
53. (2r,3r)-trans-dihydroquercetin
54. Brn 0093548
55. (2r,3r)-taxifolin
56. Unii-eas93sc1vs
57. (+/-)-trans Taxifolin
58. Schembl39786
59. 5-18-05-00451 (beilstein Handbook Reference)
60. Mls000759539
61. Mls001066341
62. Mls001074712
63. Mls001424044
64. Mls002153142
65. Bidd:er0483
66. Taxifolin, Analytical Standard
67. Flavone, 2,3-dihydro-3,3',4',5,7-pentahydroxy-
68. Dihydroquercetin [inci]
69. Megxp0_000741
70. Dtxsid8022450
71. Acon1_000239
72. Dihydroquercetin [who-dd]
73. Bdbm212435
74. Dtxsid301017215
75. Hms2051m22
76. Hms2234l14
77. Hy-n0136
78. Ac-935
79. S2366
80. Akos015965399
81. Zinc100018343
82. Ccg-100909
83. Cs-3365
84. Db02224
85. Ks-1312
86. Nc00159
87. Ncgc00016024-08
88. Ncgc00180750-01
89. 20254-28-8
90. As-17723
91. Dihydroquercetin, (+)-(2r,3r)-
92. Sw197539-2
93. T3666
94. C01617
95. (2r,3r)-3,5,7,3',4'-pentahydroxyflavanone
96. Q412191
97. Sr-01000759385
98. Flavanone, 3,3',4',5,7-pentahydroxy-, (r,r)-
99. J-011205
100. Sr-01000759385-6
101. Taxifolin, Primary Pharmaceutical Reference Standard
102. F40ab773-26fa-4112-a46d-dc970af64bc1
103. Flavanone, 3,3',4',5,7-pentahydroxy-, (2r)-trans-
104. Flavanone, 3,3',4',5,7-pentahydroxy-, Trans-(+)-
105. (+)-taxifolin (constituent Of Maritime Pine) [dsc]
106. (+)-taxifolin (constituent Of Milk Thistle) [dsc]
107. Flavanone, 3,3',4',5,7-pentahydroxy-, (2r,3r)-(+)-
108. 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4h-chromen-4-one, (2r-trans)-
109. 4h-1-benzopyran-4-one,2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-,(2r,3r)-rel-
| Molecular Weight | 304.25 g/mol |
|---|---|
| Molecular Formula | C15H12O7 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | 304.05830272 g/mol |
| Monoisotopic Mass | 304.05830272 g/mol |
| Topological Polar Surface Area | 127 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 428 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
