


1. 127502-06-1
2. 3j0kpb596q
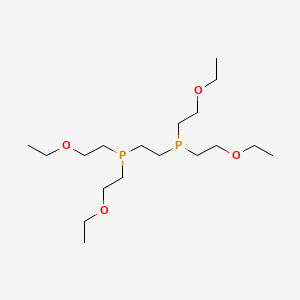
3. 3,12-dioxa-6,9-diphosphatetradecane, 6,9-bis(2-ethoxyethyl)-
4. Ethylenebis(bis(2-ethoxyethyl)phosphine)
5. P-53
6. 2-[bis(2-ethoxyethyl)phosphanyl]ethyl-bis(2-ethoxyethyl)phosphane
7. P53
8. Unii-3j0kpb596q
9. Tetrofosmin [usan:inn:ban:jan]
10. P 53
11. Tetrofosmin [ii]
12. Tetrofosmin [mi]
13. Tetrofosmin [inn]
14. Tetrofosmin [jan]
15. Tetrofosmin [usan]
16. Tetrofosmin [who-dd]
17. Schembl136150
18. Tetrofosmin (jan/usan/inn)
19. Chembl1615784
20. Dtxsid70155591
21. Chebi:135598
22. Zinc3780929
23. Db11180
24. D06094
25. Q27257279
| Molecular Weight | 382.5 g/mol |
|---|---|
| Molecular Formula | C18H40O4P2 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 19 |
| Exact Mass | 382.24018375 g/mol |
| Monoisotopic Mass | 382.24018375 g/mol |
| Topological Polar Surface Area | 36.9 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 201 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | Myoview |
| Drug Label | The MYOVIEW kit is supplied as a pack of five vials for use in the preparation of a technetium Tc99m tetrofosmin intravenous injection to be used for the scintigraphic delineation of regions of reversible myocardial ischemia in the presence |
| Active Ingredient | Technetium tc-99m tetrofosmin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | n/a |
| Market Status | Prescription |
| Company | Ge Healthcare |
Tetrofosmin is indicated to be used as a complex with technetium Tc-99m for scintigraphic imaging of the myocardium following separate administrations under exercise and/or resting conditions. It helps in the delineation of regions of reversible myocardial ischemia in absence of infarcted myocardium. This complex is also used for scintigraphic imaging of the myocardium to identify changes in perfusion induced by pharmacologic stress in patients with known or suspected coronary artery disease. This complex is indicated for the assessment of left ventricular function in patients evaluated for heart disease.
FDA Label
Refer to [DB09160]
Absorption
After intravenous administration, tetrofosmin is rapidly cleared from the blood and taken up by the heart, skeletal muscle, liver, spleen and kidneys.
Route of Elimination
Refer to [DB09160]
Volume of Distribution
This pharmacokinetic property has not been fully studied.
Clearance
Refer to [DB09160]
This pharmacokinetic property has not been fully studied.
Refer to [DB09160]
Tetrofosmin normal biodistribution makes it suitable to be used as a myocardial agent as it is uptaken by the myocardial tissue and it presents a very low redistribution after 3-4 hours of administration. After distribution, tetrofosmin is a lipophilic cationic agent which is passively diffused and accumulated in viable myocardial tissue.