

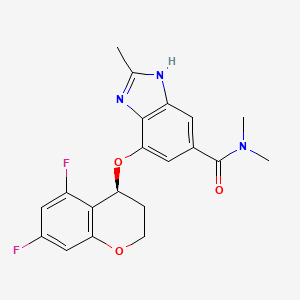

1. (s)-4-((5,7-difluorochroman-4-yl)oxy)-n,n,2-trimethyl-1h-benzo(d)imidazole-6-carboxamide
2. 1h-benzimidazole-5-carboxamide, 7-(((4s)-5,7-difluoro-3,4-dihydro-2h-1-benzopyran-4-yl)oxy)-n,n,2-trimethyl-
3. 7-(((4s)-5,7-difluoro-3,4-dihydro-2h-chromen-4-yl)oxy)-n,n,2-trimethyl-1h-benzimidazole-5-carboxamide
1. 942195-55-3
2. Tegoprazan [inn]
3. W017g7if4s
4. Lxi-15028
5. (s)-4-((5,7-difluorochroman-4-yl)oxy)-n,n,2-trimethyl-1h-benzo[d]imidazole-6-carboxamide
6. 1h-benzimidazole-5-carboxamide, 7-(((4s)-5,7-difluoro-3,4-dihydro-2h-1-benzopyran-4-yl)oxy)-n,n,2-trimethyl-
7. Unii-w017g7if4s
8. Emixustat Hcl
9. (s)-4-((5,7-difluorochroman-4-yl)oxy)-n,n,2-trimethyl-1h-benzo(d)imidazole-6-carboxamide
10. 7-[[(4s)-5,7-difluoro-3,4-dihydro-2h-chromen-4-yl]oxy]-n,n,2-trimethyl-3h-benzimidazole-5-carboxamide
11. K-cab
12. Tegoprazan [who-dd]
13. Schembl2687723
14. Chembl4297583
15. Schembl19236298
16. Gtpl12008
17. (s)-7-((5,7-difluorochroman-4-yl)oxy)-n,n,2-trimethyl-1h-benzo(d)imidazole-5-carboxamide
18. Ex-a4304
19. Cj12420
20. Ac-36576
21. As-84160
22. Cj-12420
23. Hy-17623
24. Cs-0014702
25. E83739
26. Cj-12420; In-a001; Lxi-15028
27. Q27292116
28. (-)-4-[((4s)-5,7-difluoro-3,4-dihydro-2h-chromen-4-yl)oxy]-n,n,2-trimethyl-1h-benzimidazole-6-carboxamide
29. 7-(((4s)-5,7-difluoro-3,4-dihydro-2h-chromen-4-yl)oxy)-n,n,2-trimethyl-1h-benzimidazole-5-carboxamide
30. 7-[[(4s)-5,7-difluoro-3,4-dihydro-2h-1-benzopyran-4-yl]oxy]-n,n,2-trimethyl-1h-benzimidazole-5-carboxamide
31. 8bn
| Molecular Weight | 387.4 g/mol |
|---|---|
| Molecular Formula | C20H19F2N3O3 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 387.13944780 g/mol |
| Monoisotopic Mass | 387.13944780 g/mol |
| Topological Polar Surface Area | 67.4 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 581 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BC - Proton pump inhibitors
A02BC09 - Tegoprazan
Tegoprazan works as a potassium-competitive acid blocker that is potent and highly selective. Its mechanism of action is different from that of the proton-pump inhibitors as this drug does not require conversion into an active form and can directly inhibit H+/K+ATPase in a reversible and K+competitive way. This is because it is an acid-resistant weak base with the ability to remain in the highly acidic canaliculi of gastric parietal cells.
