
1. Incivek
2. Vx 950
3. Vx-950
4. Vx950 Cpd
1. 402957-28-2
2. Vx-950
3. Incivek
4. Telaprevir (vx-950)
5. Incivo
6. Mp-424
7. Vx 950
8. Ly-570310
9. Telavic
10. Mp 424
11. Vrt 111950
12. Vrt-111950
13. Ly 570310
14. Chembl231813
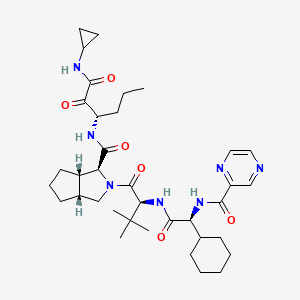
15. (1s,3ar,6as)-2-((s)-2-((s)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-n-((s)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide
16. Chebi:68595
17. 655m5o3w0u
18. (3s,3as,6ar)-2-[(2s)-2-[[(2s)-2-cyclohexyl-2-(pyrazine-2-carbonylamino)acetyl]amino]-3,3-dimethylbutanoyl]-n-[(3s)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl]-3,3a,4,5,6,6a-hexahydro-1h-cyclopenta[c]pyrrole-3-carboxamide
19. (1s,3ar,6as)-2-((s)-2-((s)-2-cyclohexyl-2-(pyrazine-6-carboxamido)acetamido)-3,3-dimethylbutanoyl)-n-((s)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)-octahydrocyclopenta[c]pyrrole-1-carboxamide
20. S-telaprevir
21. (1s,3ar,6as)-(2s)-2-cyclohexyl-n-(pyrazinylcarbonyl)glycyl-3-methyl-l-valyl-n-((1s)-1-((cyclopropylamino)oxoacetyl)butyl)octahydrocyclopenta(c)pyrrole-1-carboxamide
22. Cyclopenta(c)pyrrole-1-carboxamide, (2s)-2-cyclohexyl-n-(pyrazinylcarbonyl)glycyl-3-methyl-l-valyl-n-((1s)-1-((cyclopropylamino)oxoacetyl)butyl)octahydro-, (1s,3ar,6as)-
23. Vx950 Cpd
24. Telaprevir [usan:inn]
25. Unii-655m5o3w0u
26. Hsdb 8125
27. Incivek(tm)
28. Telaprevir-[d4]
29. 569364-34-7
30. Incivek (tn)
31. Incivo (tn)
32. Telaprevir,vx-950
33. Telaprevir [mi]
34. Telaprevir [inn]
35. Telaprevir [jan]
36. (1s,3ar,6as)-2-[(2s)-2-[[(2s)-2-cyclohexyl-2-(pyrazine-2-carbonylamino)acetyl]amino]-3,3-dimethylbutanoyl]-n-[(3s)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl]-3,3a,4,5,6,6a-hexahydro-1h-cyclopenta[c]pyrrole-1-carboxamide
37. Telaprevir [usan]
38. Vx-950 (telaprevir)
39. Telaprevir [vandf]
40. Telaprevir [mart.]
41. Telaprevir [who-dd]
42. Schembl183996
43. Telaprevir (jan/usan/inn)
44. Gtpl7871
45. Telaprevir [orange Book]
46. Dtxsid40193304
47. Ex-a006
48. Aids213006
49. Aids-213006
50. Zinc3992480
51. Bdbm50326056
52. Fd7166
53. Mfcd11616089
54. S1538
55. Vrt111950
56. Akos005145815
57. Ccg-270366
58. Cs-0285
59. Db05521
60. Ncgc00346545-03
61. (3s,3as,6ar)-2-[(2s)-2-[[(2s)-2-cyclohexyl-2-(pyrazine-2-carbonylamino)acetyl]amino]-3,3-dimethyl-butanoyl]-n-[(1s)-1-[2-(cyclopropylamino)-2-oxo-acetyl]butyl]-3,3a,4,5,6,6a-hexahydro-1h-cyclopenta[c]pyrrole-3-carboxamide
62. As-16995
63. Hy-10235
64. Wo-00218369
65. D09012
66. 957t282
67. Q408557
68. Q-101417
69. (1s,3ar,6as)-(2s)-2-cyclohexyl-n-(2-pyrazinylcarbonyl)glycyl-3-methyl-l-valyl-n-[(1s)-1-[2-(cyclopropylamino)-2-oxoacetyl]butyl]octahydrocyclopenta[c]pyrrole-1-carboxamide
70. (1s,3ar,6as)-2-((2s)-2-(((2s)-cyclohexyl((pyrazinylcarbonyl)amino)acetyl)amino)-3,3-dimethylbutanoyl)-n-((1s)-1-((cyclopropylamino)oxoacetyl)butyl) Octahydrocyclopenta(c)pyrrole-1-carboxamide
71. (1s,3ar,6as)-2-((s)-2-((s)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-n-((s)-1-(cyclopropylamino)-1,2-dioxo-hexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide
72. (1s,3ar,6as)-2-((s)-2-((s)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-n-((s)-1-(cydopropylamino)-1,2-dioxo-hexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide
73. (1s,3ar,6as)-2-((s)-2-((s)-2-cyclohexyl-2-(pyrazine-3-carboxamido)acetamido)-3,3-dimethylbutanoyl)-n-((s)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)-octahydrocyclopenta[c]pyrrole-1-carboxamide
74. (1s,3ar,6as)-2-[(2s)-2-({(2s)-2-cyclohexyl-2-[(pyrazin-2-ylcarbonyl)amino]acetyl}amino)-3,3-dimethylbutanoyl]-n-[(3s)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl]octahydrocyclopenta[c]pyrrole-1-carboxamide
75. (1s,3ar,6as)-n-((s)-2-((s)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-n-((s)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide
76. (3s)-3-{[(1s,3ar,6as)-2-[(2s)-2-[(2s)-2-cyclohexyl-2-(pyrazin-2-ylformamido)acetamido]-3,3-dimethylbutanoyl]-octahydrocyclopenta[c]pyrrol-1-yl]formamido}-n-cyclopropyl-2-oxohexanamide
77. 2-(2-{2-cyclohexyl-2-[(pyrazine-2-carbonyl)-amino]-acetylamino}-3,3-dimethyl-butyryl)-octahydro-cyclopenta[c]pyrrole-1-carboxylic Acid (1-cyclopropylaminooxalyl-butyl)-amide
| Molecular Weight | 679.8 g/mol |
|---|---|
| Molecular Formula | C36H53N7O6 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 14 |
| Exact Mass | 679.40573244 g/mol |
| Monoisotopic Mass | 679.40573244 g/mol |
| Topological Polar Surface Area | 180 Ų |
| Heavy Atom Count | 49 |
| Formal Charge | 0 |
| Complexity | 1240 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Incivek |
| PubMed Health | Telaprevir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | INCIVEK (telaprevir) is an inhibitor of the HCV NS3/4A protease.The IUPAC name for telaprevir is (1S,3aR,6aS)-2-[(2S)-2-({(2S)-2-cyclohexyl-2-[(pyrazin-2-ylcarbonyl)amino]acetyl}amino)-3,3-dimethylbutanoyl]-N-[(3S)-1-(cyclopropylamino)-1,2-dioxohexan... |
| Active Ingredient | Telaprevir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 375mg |
| Market Status | Prescription |
| Company | Vertex Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Incivek |
| PubMed Health | Telaprevir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | INCIVEK (telaprevir) is an inhibitor of the HCV NS3/4A protease.The IUPAC name for telaprevir is (1S,3aR,6aS)-2-[(2S)-2-({(2S)-2-cyclohexyl-2-[(pyrazin-2-ylcarbonyl)amino]acetyl}amino)-3,3-dimethylbutanoyl]-N-[(3S)-1-(cyclopropylamino)-1,2-dioxohexan... |
| Active Ingredient | Telaprevir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 375mg |
| Market Status | Prescription |
| Company | Vertex Pharms |
Oligopeptides
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
INCIVEK (telaprevir), in combination with peginterferon alfa and ribavirin, is indicated for the treatment of genotype 1 chronic hepatitis C in adult patients with compensated liver disease, including cirrhosis, who are treatment-naive or who have previously been treated with interferon-based treatment, including prior null responders, partial responders, and relapsers. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for INCIVEK (telaprevir) tablet, film coated (December 2012). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ed0e4f33-cf21-4fe3-918d-1d5b3a23eee4
Fatal and non-fatal serious skin reactions, including Stevens Johnson Syndrome (SJS), Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), and Toxic Epidermal Necrolysis (TEN), have been reported in patients treated with INCIVEK combination treatment. Fatal cases have been reported in patients with progressive rash and systemic symptoms who continued to receive INCIVEK combination treatment after a serious skin reaction was identified. For serious skin reactions, including rash with systemic symptoms or a progressive severe rash, INCIVEK, peginterferon alfa, and ribavirin must be discontinued immediately. Discontinuing other medications known to be associated with serious skin reactions should be considered. Patients should be promptly referred for urgent medical care.
US Natl Inst Health; DailyMed. Current Medication Information for INCIVEK (telaprevir) tablet, film coated (December 2012). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ed0e4f33-cf21-4fe3-918d-1d5b3a23eee4
Rash developed in 56% of patients receiving telaprevir during controlled clinical trials. Severe rash (e.g., generalized rash or rash with vesicles or bullae or ulcerations other than SJS) was reported in 4% of patients receiving telaprevir in conjunction with peginterferon alfa and ribavirin compared with less than 1% of patients receiving peginterferon alfa and ribavirin without telaprevir. Rash frequently was observed during the first 4 weeks of telaprevir treatment, but can occur at any time. Rash generally improves when telaprevir therapy is completed or discontinued; complete resolution may take weeks.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 853
If a serious skin reaction occurs, telaprevir, peginterferon alfa, and ribavirin should be immediately discontinued and the patient promptly referred for urgent medical care. Patients with mild to moderate rash should be monitored for progression of rash or development of systemic symptoms. If rash progresses and becomes severe or if systemic symptoms develop, telaprevir should be discontinued; peginterferon alfa and ribavirin may be continued.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 853
Telaprevir dosage should not be reduced and telaprevir should not be restarted if it was discontinued because of rash. If improvement is not observed within 7 days of discontinuing telaprevir, sequential or simultaneous interruption or discontinuance of peginterferon alfa and/or ribavirin should be considered. If medically indicated, earlier interruption or discontinuance of peginterferon alfa and ribavirin should be considered.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 853
For more Drug Warnings (Complete) data for Telaprevir (18 total), please visit the HSDB record page.
Telaprevir, when used in combination with [DB00811], [DB00008], and [DB00022] is indicated for use in the treatment of chronic HCV genotype 1 infection in adults.
FDA Label
Incivo, in combination with peginterferon alfa and ribavirin, is indicated for the treatment of genotype-1 chronic hepatitis C in adult patients with compensated liver disease (including cirrhosis):
- who are treatment nave;
- who have previously been treated with interferon alfa (pegylated or non-pegylated) alone or in combination with ribavirin, including relapsers, partial responders and null responders.
Telaprevir is classified as a direct-acting antiviral (DAA) and prevents viral replication in HCV genotype 1.
J05AE
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AP - Antivirals for treatment of hcv infections
J05AP02 - Telaprevir
Absorption
Telaprevir reaches peak plasma concentration 4-5hours after administration. Absolute bioavailability has not been determined. When taken with a normal fat meal (21g of fat), exposure increases by 235% compared to fasting conditions. With low (3.6g of fat) and high fat (56g of fat) meals, exposure increased 117% and 330% respectively.
Route of Elimination
Telaprevir is mainly eliminated in the feces (82%) with a smaller amount eliminated via expiration (9%) and very little in the urine (1%). 31.9% and 18.8% of drug in the feces was present as the parent compound and R-diastereomer of the parent compound respectively.
Volume of Distribution
The estimated apparent volume of distribution for Telapravir is 252 litres with an inter-individual variability of 72%.
Clearance
Telaprevir has an estimated aparent total body clearance of 32.4 liters per hour with an interindividual variability of 27.2%.
The pharmacokinetic properties of telaprevir have been evaluated in healthy adult subjects and in subjects with chronic hepatitis C. Following multiple doses of telaprevir (750 mg every 8 hr) in combination with peginterferon alfa and ribavirin in treatment-naive subjects with genotype 1 chronic hepatitis C, mean (SD) Cmax was 3510 (1280) ng/mL, Cmin was 2030 (930) ng/mL, and AUC8h was 22,300 (8650) ng.hr/mL.
US Natl Inst Health; DailyMed. Current Medication Information for INCIVEK (telaprevir) tablet, film coated (December 2012). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ed0e4f33-cf21-4fe3-918d-1d5b3a23eee4
Telaprevir is orally available, most likely absorbed in the small intestine, with no evidence for absorption in the colon. Maximum plasma concentrations after a single dose of telaprevir are generally achieved after 4 to 5 hours.
US Natl Inst Health; DailyMed. Current Medication Information for INCIVEK (telaprevir) tablet, film coated (December 2012). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ed0e4f33-cf21-4fe3-918d-1d5b3a23eee4
Telaprevir is a substrate for and inhibitor of P-glycoprotein transport.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 855
The systemic exposure (AUC) to telaprevir was increased by 237% when telaprevir was administered with a standard fat meal (containing 533 kcal and 21 g fat) compared to when telaprevir was administered under fasting conditions. In addition, the type of meal significantly affects exposure to telaprevir. Relative to fasting, when telaprevir was administered with a low-fat meal (249 kcal, 3.6 g fat) and a high-fat meal (928 kcal, 56 g fat), the systemic exposure (AUC) to telaprevir was increased by approximately 117% and 330%, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for INCIVEK (telaprevir) tablet, film coated (December 2012). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ed0e4f33-cf21-4fe3-918d-1d5b3a23eee4
For more Absorption, Distribution and Excretion (Complete) data for Telaprevir (13 total), please visit the HSDB record page.
Telaprevir is extensively metabolized via hydrolysis, oxidation, and reduction. The major metabolites of Telaprevir are pyrazinoic acid, a metabolite that underwent reduction at the -ketoamide bond, and the R-diastereomer of telaprevir which is 30-fold less active than the parent compound were found to be the predominant metabolites. The primary enzyme involved in the metabolism of Telaprevir is CYP3A4. Some metabolism is performed by aldo-keto reductases and other reductases.
Telaprevir is extensively metabolized in the liver, involving hydrolysis, oxidation, and reduction. Multiple metabolites were detected in feces, plasma, and urine. After repeated oral administration, the R-diastereomer of telaprevir (30-fold less active), pyrazinoic acid, and a metabolite that underwent reduction at the alpha-ketoamide bond of telaprevir (not active) were found to be the predominant metabolites of telaprevir.
US Natl Inst Health; DailyMed. Current Medication Information for INCIVEK (telaprevir) tablet, film coated (December 2012). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ed0e4f33-cf21-4fe3-918d-1d5b3a23eee4
Telaprevir has a half-life of elimination of 4.0-4.7 hours after a single dose and an effective half life of 9-11 hours at steady state.
The mean elimination half-life after single-dose oral administration of telaprevir 750 mg typically ranged from about 4.0 to 4.7 hours. At steady state, the effective half-life is about 9 to 11 hours.
US Natl Inst Health; DailyMed. Current Medication Information for INCIVEK (telaprevir) tablet, film coated (December 2012). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ed0e4f33-cf21-4fe3-918d-1d5b3a23eee4
Telaprevir is a NS3/4a protease inhibitor used to inhibit viral HCV replication. NS3/4a protease is an integral part of viral replication and mediates the cleavage the virally encoded polyprotein to mature proteins (NS4A, NS4B, NS5A and NS5B). Telaprevir inhibits NS3/4A with an IC50 of 10nM.
Telaprevir is a peptidomimetic, selective hepatitis C virus (HCV) nonstructural 3/4A (NS3/4A) protease inhibitor. The drug is a direct-acting antiviral (DAA) agent with activity against HCV. Telaprevir contains an alpha-ketoamide functional group that covalently and reversibly binds the active serine site of HCV NS3/4 protease. By blocking proteolytic cleavage of NS4A, NS4B, NS5A, and NS5B from the HCV-encoded polyprotein, the drug inhibits HCV replication. Telaprevir has in vitro activity against HCV genotypes 1a, 1b, and 2, but is less active against genotypes 3a and 4a.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 855
Telaprevir is an inhibitor of the HCV NS3/4A serine protease, necessary for the proteolytic cleavage of the HCV encoded polyprotein into mature forms of the NS4A, NS4B, NS5A and NS5B proteins and essential for viral replication. In a biochemical assay, telaprevir inhibited the proteolytic activity of the recombinant HCV NS3 protease domain with an IC50 value of 10 nM.
US Natl Inst Health; DailyMed. Current Medication Information for INCIVEK (telaprevir) tablet, film coated (December 2012). Available from, as of March 12, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ed0e4f33-cf21-4fe3-918d-1d5b3a23eee4