


1. Azd1722
2. Rdx5791
1. 1234423-95-0
2. Azd1722
3. Azd-1722
4. Tenapanor Free Base
5. Rdx-5791
6. Azd 1722
7. Rdx 5791
8. Ibsrela
9. Rdx5791
10. Wyd79216a6
11. 1234423-95-0 (free Base)
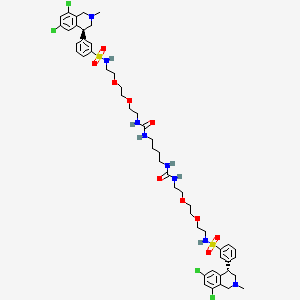
12. 3-((s)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)-n-(26-((3-((s)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenyl)sulfonamido)-10,17-dioxo-3,6,21,24-tetraoxa-9,11,16,18-tetraazahexacosyl)benzenesulfonamide
13. N,n'-(10,17,-dioxo-3,6,21,24-tetraoxa-9,11,16,18-tetraazahexacosane-1,26-diyl)bis(((4s)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)benzenesulfonamide)
14. Tenapanor [inn]
15. 1-[2-[2-[2-[[3-[(4s)-6,8-dichloro-2-methyl-3,4-dihydro-1h-isoquinolin-4-yl]phenyl]sulfonylamino]ethoxy]ethoxy]ethyl]-3-[4-[2-[2-[2-[[3-[(4s)-6,8-dichloro-2-methyl-3,4-dihydro-1h-isoquinolin-4-yl]phenyl]sulfonylamino]ethoxy]ethoxy]ethylcarbamoylamino]butyl]urea
16. Tenapanor [usan:inn]
17. Unii-wyd79216a6
18. Khk-7791
19. Tenapanor [mi]
20. Tenapanor (usan/inn)
21. Tenapanor [usan]
22. Tenapanor [who-dd]
23. Gtpl8449
24. Chembl3304485
25. Schembl15267600
26. Dtxsid40154016
27. Khk7791
28. Bdbm381823
29. Rdx013 Component Tenapanor
30. Bcp24892
31. Bcp28554
32. Ex-a2506
33. Rdx-013 Component Tenapanor
34. Mfcd28386333
35. Tenapanor; Azd1722; Rdx5791
36. Us10272079, Compound 002
37. Us10272079, Compound 180
38. Akos037648586
39. Tenapanor Component Of Rdx013
40. Cs-6273
41. Db11761
42. Tenapanor Component Of Rdx-013
43. Ac-36104
44. Bs-14732
45. Bt178667
46. Hy-15991
47. J3.655.031g
48. D11652
49. A929505
50. Q17122912
51. Azd 1722;azd1722;azd-1722;rdx5791;rdx 5791;rdx-5791
52. Azd-1722; Azd 1722; Azd1722; Rdx 5791; Rdx-5791; Rdx5791
53. 12,15-dioxa-2,7,9-triazaheptadecanamide, 17-(((3-((4s)-6,8-dichloro-1,2,3,4-tetrahydro-2-methyl-4-isoquinolinyl)phenyl)sulfonyl)amino)-n-(2-(2-(2-(((3-((4s)-6,8-dichloro-1,2,3,4-tetrahydro-2-methyl-4-isoquinolinyl)phenyl)sulfonyl)amino)ethoxy)ethoxy)ethyl)-8-oxo-
54. 17-[[[3-[(4s)-6,8-dichloro-1,2,3,4-tetrahydro-2-methyl-4-isoquinolinyl]phenyl]sulfonyl]amino]-n-[2-[2-[2-[[[3-[(4s)-6,8-dichloro-1,2,3,4-tetrahydro-2-methyl-4-isoquinolinyl]phenyl]sulfonyl]amino]ethoxy]ethoxy]ethyl]-8-oxo-12,15-dioxa-2,7,9-triazaheptadecanamide
| Molecular Weight | 1145.0 g/mol |
|---|---|
| Molecular Formula | C50H66Cl4N8O10S2 |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 29 |
| Exact Mass | 1144.306793 g/mol |
| Monoisotopic Mass | 1142.309743 g/mol |
| Topological Polar Surface Area | 235 Ų |
| Heavy Atom Count | 74 |
| Formal Charge | 0 |
| Complexity | 1770 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tenapanor is indicated for the treatment of constipation-predominant irritable bowel syndrome (IBS-C) in adults. It is also currently being investigated as a treatment for hyperphosphatemia in chronic kidney disease patients undergoing dialysis (NCT02081534 and NCT02675998).
Through the inhibition of dietary sodium absorption tenapanor causes an increase in water secretion into the intestines, thereby decreasing transit time and softening stool consistency.
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AX - Other drugs for constipation
A06AX08 - Tenapanor
Absorption
Tenapanor undergoes very minimal systemic absorption following oral administration. During clinical trials, plasma concentrations were below the limit of quantitation (i.e. less than 0.5 ng/mL) in the majority of samples from healthy subjects - for this reason, typical pharmacokinetic values related to absorption such as AUC and Cmax were unable to be ascertained. The effects of tenapanor are greatest when administered 5 to 10 minutes before meals.
Route of Elimination
Following administration of a radio labeled dose of tenapanor, 70% of the radioactivity was excreted in the feces within 120 hours of administration and 79% within 240 hours. Approximately 65% of the total dose is excreted as unchanged parent drug within 144 hours of administration. Only 9% of the administered dose was found in the urine, existing primarily as metabolites. Tenapanor's M1 metabolite is excreted unchanged in the urine and accounts for approximately 1.5% of the total dose within 144 hours of administration.
The majority of tenapanor's metabolism to its primary metabolite, M1, is catalyzed via CYP3A4/5. Exposure of tenapanor to hepatic CYP enzymes is likely limited due to its minimal systemic absorption, so its metabolism may be due to intestinal CYP enzyme activity. The M1 metabolite of tenapanor is a P-glycoprotein substrate and, in contrast to its parent drug, can be detected in plasma, reaching a Cmax of approximately 15 ng/mL at steady state. It is not considered active against NHE3.
Tenapanor's FDA label states that its half-life could not be determined during clinical trials due to its minimal systemic absorption resulting in plasma concentrations below the limit of quantitation (i.e. less than 0.5 ng/mL).
Tenapanor is a locally-acting small molecule inhibitor of the sodium/hydrogen exchanger isoform 3 (NHE3), an antiporter expressed on the apical surface of enterocytes in the small intestine and colon which is involved in sodium-fluid homeostasis. By inhibiting this antiporter tenapanor causes retention of sodium within the lumen of the intestine - this results in an osmotic gradient that draws water into the lumen and softens stool consistency. There is some evidence that tenapanor can inhibit the uptake of dietary phosphorus in the gastrointestinal tract, though the exact mechanism of this activity has yet to be elucidated.
